Introduction
Molar masses that are calculated to be too high or too low than the actual molar mass are deemed abnormal molar mass. They are calculated using the colligative characteristics. Among the colloquial features are a higher osmotic pressure, a lower vapour pressure, a lower freezing point, and a higher boiling point. Van’t Hoff factor is used in such cases to find the actual molar mass.
What is the Van’t Hoff Factor?
- Because the actual molar mass of a substance is not always equal to the sum of the atomic masses of the atoms making up the substance, a quantity called the Vant Hoff factor is employed to account for this discrepancy. This occurs because the arrangement of atoms in a substance is not constant. To make sense of this variation in setup, the Vant Hoff factor is employed.
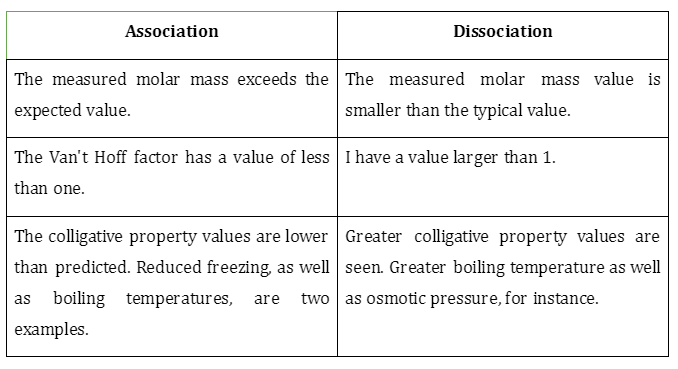
- It is denoted by the symbol i. A material’s degree of association or dissociation in a fluid is the relevant factor. When something that isn’t electrolytic dissolves in water, the value of i will typically always equal 1. When dissolving in water, the number of ions (i) remains constant regardless of how many atoms (N) of the substance are dissolved.
- Jacobus Henricus Van’t Hoff, the first recipient of the Nobel Prize in Chemistry, is honoured with the naming of this constant. In the case of electrolytic fluids, it is important to note that the measured factor will be smaller than predicted. A greater divergence is seen at higher ion charges.
Effects of Association/Dissociation
- The combining of 2 or more substances to produce a single object is known as an association. Carboxylic acids show a high degree of association through hydrogen bonding.
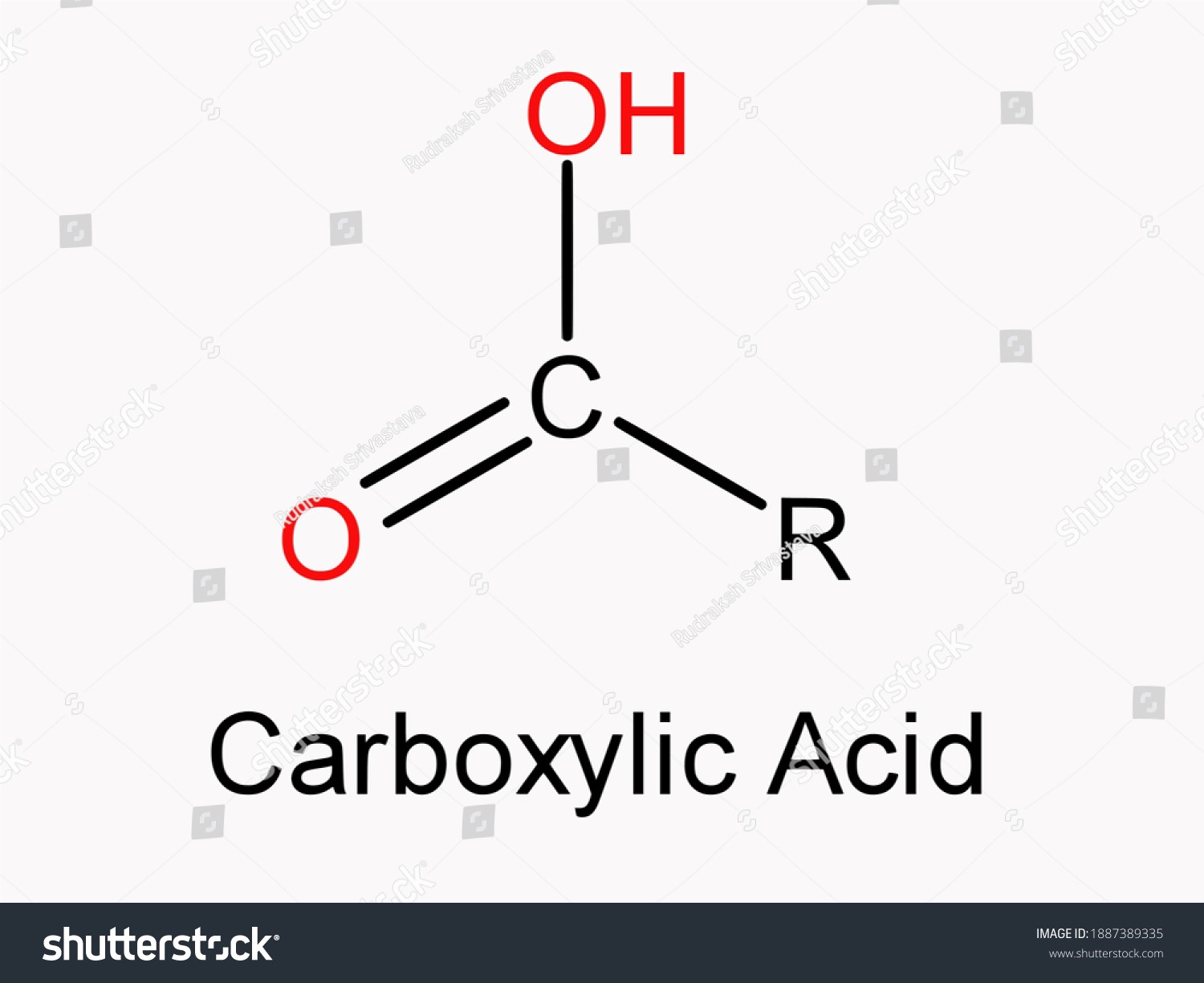
- Dissociation is the process by which a substance is split up into its constituent ions. Sodium chloride breaks down into Cl- and Na+ ions when exposed to water.
- The following table summarizes the consequences of a solute’s dissociation or even association on the Van’t Hoff factor.
Abnormal Molar Masses
An irregularity in molecular mass occurs in the following cases:
- The dissolution of solute components into many ions increases the total particle content and hence enhances the colligative features of the mixture.
- Due to the inverse relationship between molar mass and colligative properties, we anticipate a smaller final outcome.
- Since the number of solute particles is reduced as a result of their interactions, colligative characteristics are also reduced. Readings of molar mass in this scenario will be higher than expected.
Summary
Molar mass is the overall no. of moles available in a solution following solute association or even dissociation. Abnormal molar mass occurs when the molar mass is less than or more than the predicted value. The Van’t Hoff factor gives accurate data on how solutes affect the colligative properties of fluids. It will always be smaller than one for solutes that show an association. The factor will be greater than one for dissociating solutes. It will be one for particles that demonstrate no association or even dissociation.
Frequently Asked Questions
1. What is the difference between the Vant Hoff factor and the activity coefficient?
Ans. The Vant Hoff factor is a measure of the degree of dissociation of a solute in a solution. The activity coefficient is a measure of the degree of ionization of a solute in a solution.
2.Does van’t hoff factor depends on solvent?
Ans. Since colligative properties depend only on the number of solute particles, there is no affect of solvent. Hence vant hoff factor does not depend on the solvent.
3. Does Vant hoff factor always have an integral value?
Ans. The van’t Hoff factor is a positive number, but it isn’t always an integer value. It is 1 for a solute that does not dissociate into ions, greater than 1 for most salts and acids, and less than 1 for solutes that form associations when dissolved.