Introduction
Colloids are a type of mixture in which one material is broken down into tiny particles (known colloidal particles) and spread throughout another. The particles are larger than those found in the solution, yet they are too small to be seen using a microscope. There are no precise size limits for colloidal particles, however, they tend to be in the 10-9m to 10-6m. Preparation of colloids can be done by two methods: dispersion and condensation methods.
What are Colloids?
Colloids are a special kind of mixture in which one substance is dispersed throughout another after being broken down into very small pieces (the “colloidal particles”). Despite being larger than the particles in the solution, they are still too small to be seen with the naked eye.
Colloidal particles can have any size from \({10^9}m\;to\;{10^6}m\) , but typically fall into that range. There are two ways to get colloids ready for use: dispersion or condensation.
Examples of Colloids
Here are several examples of colloids:
- Solid Sols- Pearl, some alloys, gemstones, etc.
- Gels- Gelatin, jelly, etc.
- Aerosols- Fog, clouds, dust, etc.
- Solid Foams- Marshmallows, Styrofoam, etc.
- Emulsions- Milk, lotion, etc.
- Foams- whipped cream, shaving cream, etc.
- Sols- Ink, shampoo, etc.
Classification of Colloids:
Based on Physical State
We can divide colloids into 8 groups:
- Foam
- Emulsion
- Solid aerosol
- Gel
- Solid sol
- Solid foam
- Aerosol
- Sol
Based on Interaction Forces
Lyophilic colloids
Solutions have a colloidal particle size in which the particles of the dispersion phase are evenly distributed and exhibit a high attraction for the dispersed phase known as lyophilic colloids. These sols are quite stable and do not coagulate quickly. Gums, and proteins, along with other lyophilic colloids, are examples.
Lyophobic colloids
Colloidal solutions wherein the dispersed medium particles have no attraction to the dispersion medium are known as lyophobic colloids. For the sake of preservation, these sols require some stabilizing chemicals.
Classification Based on Properties of Sol Particle
Multi Molecular Colloids
When a substance disintegrates, atoms/ molecules clump together to produce colloidal particles. Multimolecular colloids are the particles that result from this process.
Macromolecular Colloids
These are macromolecular colloids, which are macromolecular substances with large molecular sizes that, when broken down, generate a size in the colloids. As a result, the macromolecules that make up the dispersed phase are usually polymers with extremely high molecular weights.
Based on Dispersion Medium
It can be categorized into four categories based on the dispersion medium:
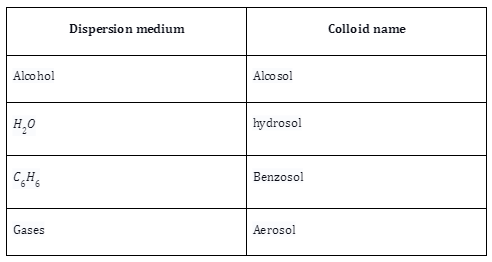
Preparation of Colloid Solutions:
Condensation method
- By exchange of solvent, An S or P solution in alcohol is emptied into \({{\bf{H}}_2}{\bf{O}}\) to form a colloid of S or P due to its limited water solubility.
- By change of physical state S or Hg, for example, can be turned into colloidal solutions by transporting their vapours through a stabiliser-laced cold water
- Chemical Methods: Double decomposition: To make \(A{s_2}S\) sol, a constant stream of \({{\bf{H}}_2}{\bf{S}}\) gas is fed through such a freezing solution of \(A{s_2}{O_3}\) . This process is continued until the sol’s yellow colour reaches its maximal intensity.
- Oxidation: When \({{\bf{H}}_2}{\bf{S}}\) is passed through \({\bf{S}}{{\bf{O}}_2}\) solution, it forms a colloidal solution.
- \({\bf{2}}{{\bf{H}}_2}{\bf{S}} + {\bf{S}}{{\bf{O}}_2} \to \;{\bf{2}}{{\bf{H}}_2}{\bf{O}} + {\bf{3S}}\)
- By excessive cooling: By freezing a water solution in an organic solvent such as ether/\(CHC{l_3}\), a colloidal ice solution can be created.
Dispersion Methods
Mechanical dispersion
A colloidal mill is used to grind the solid and liquid together. The colloidal mill is made up of two steel plates that are closely touching and revolving at high speeds in opposite directions. This process is used to create colloidal graphite and printing inks.
Bredig’s Arc Method
Colloids of metals such as gold, platinum, as well as silver can be made using this method. Under the surface of the water with a stabilizing substance, such as a little amount of alkali, an arc is produced between metal electrodes.
Peptisation
Peptisation is the activity of an electrolyte in a solution to disperse a precipitated substance into a colloidal solution. A peptizing agent is an electrolyte employed.
Purification of Colloids:
Dialysis:
The principle behind dialysis is that colloidal particles cannot flow through parchment or cellophane membranes, but electrolyte ions may. Dialysis is the technique of removing colloidal particles from contaminants by diffusing them through a suitable membrane.
Electro-dialysis:
When an electric field is created between the electrodes, the ions in the electrolyte that are present as impurities diffuse rapidly approaching oppositely charged electrodes. The electric field is needed for this.
Ultrafiltration:
The pores of typical filter paper are large enough to allow both impurities and colloidal particles to pass through. Ultrafilters are the resultant filter sheets. Ultrafiltration is the process of filtering through ultrafilters.
Commercial Applications of Colloid
In pharmacy:
Because colloidal drugs are easily absorbed by biological tissues, they are much more efficacious.
In environment:
Purification of water: Some electrolytes, like alum, can be employed to precipitate colloidal pollutants in water.
In industrial products:
Rubber industry: Latex is made up of negatively charged rubber particles suspended in a colloidal fluid. Rubber can be made from latex via coagulation.
In the field of defence:
Colloids are employed in defence in the form of smoke in smoke screens to disguise anything in the military. Colloids are employed in rocket technology. A colloid thruster is used in rockets.
Summary
Colloids, then, are a type of mixture in which one component is spread throughout another substance after being broken down into extremely small particles. Colloidal solutions contain particles of sizes between 1 nanometer and 500 nanometers, which are in between those of a real solution and those of a pure solution. It is typically categorised as a 2-phase, heterogeneous/homogeneous system, depending on the conditions. Colloids are dispersed mixtures in which the suspended particles do not sink to the bottom.
Frequently Asked Questions
1. What is Ultracentrifugation?
Ans. It is the process of using centrifugal force to separate colloidal particles from contaminants. The impure sol is collected in a tube, which is then placed in an ultracentrifuge.
2. Why are the colligative properties of colloids of low order?
Ans. Because colloidal particles are larger aggregates, the particles in colloids are smaller than in a true solution. As a result, when compared to true solution values at similar proportions, measurements of colligative qualities are of low order.
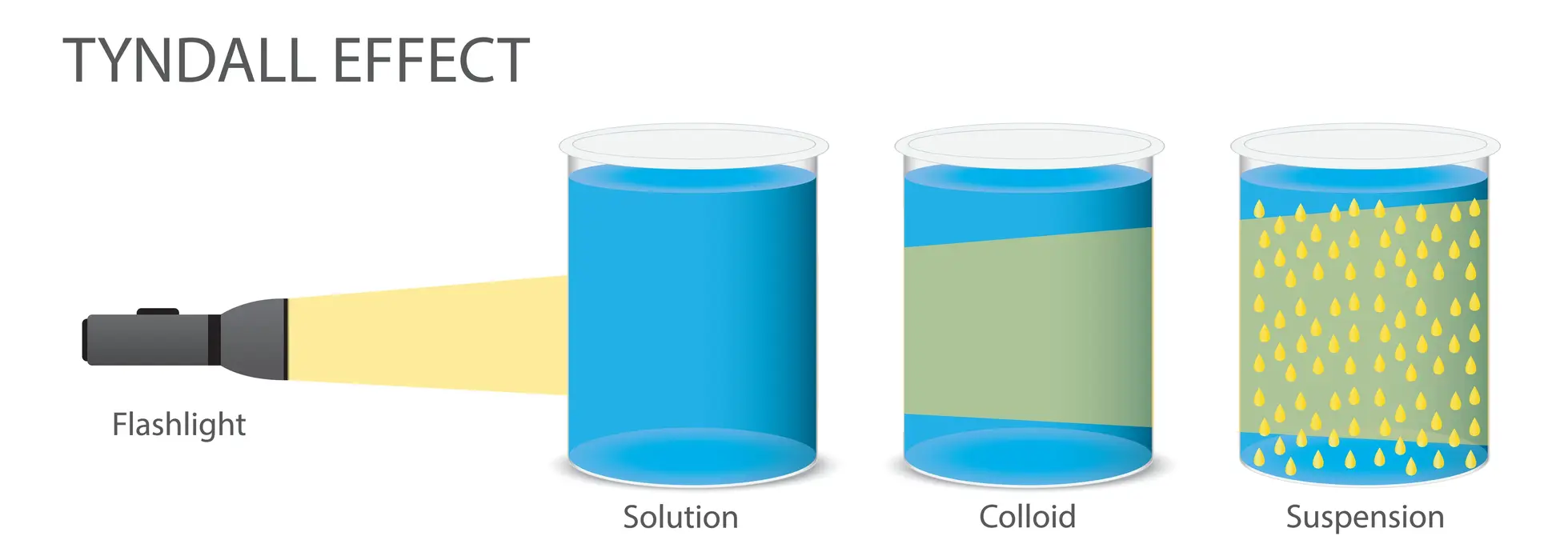
3. What is the Tyndall effect?
Ans. The Tyndall effect confirms the colloidal solution’s heterogeneous character. As light travels through a sol, it is scattered by particles, revealing its route and called the Tyndall effect.