Introduction
The distance between the nucleus’s core and the valence shell/outermost shell, known as the atomic radius of an element, serves as a benchmark for the size of its atom. The periodic table shows that the atomic size increases as you move down it and decreases as you move from left to right. The reason for this is that as you move down the group, the number of shells increases, the screening effect multiplies, and the force of attraction weakens, causing the atomic radius to increase. Additionally, the nucleus’ protons increase as you move from left to right, drawing electrons in and shrinking the atomic radius in the process.
Basic understanding of Atomic Radius
The atomic radius is typically defined as the total distance from an atom’s nucleus to its outermost electron orbital. It can be expressed more simply as something resembling a circle’s radius, with the nucleus serving as the circle’s centre and the electron’s furthest orbital serving as the circle’s edge.
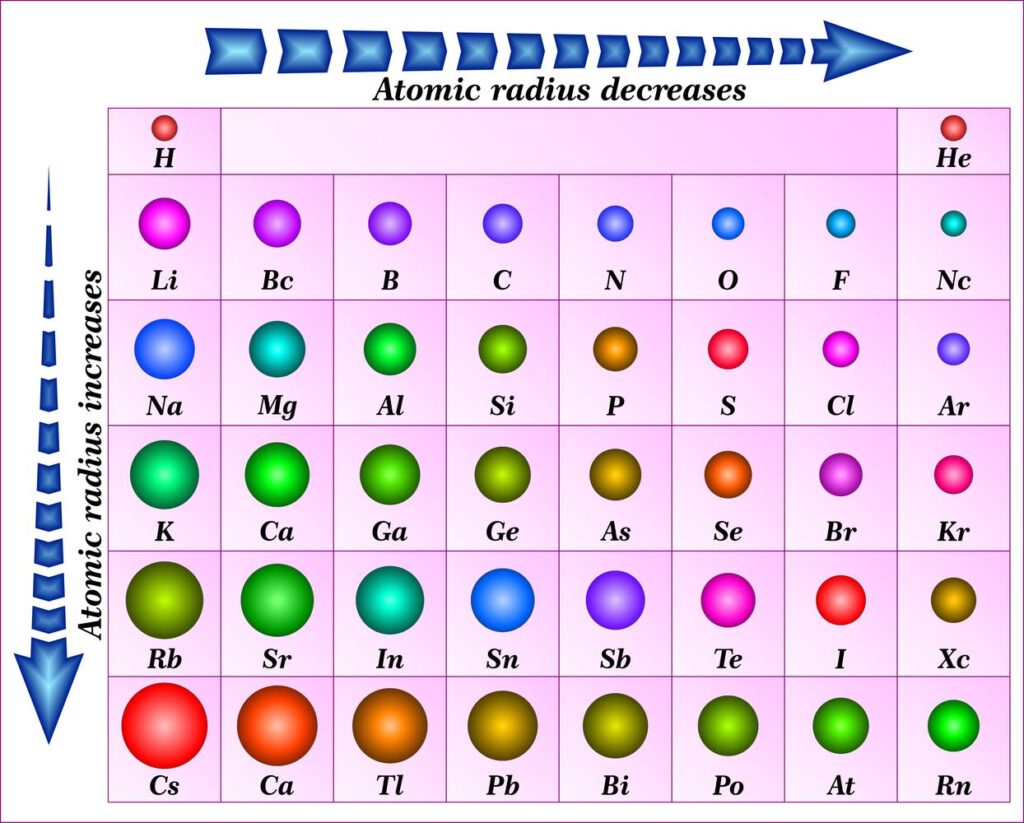
What Are the Trends in Atomic Radius? Why Do They Occur?
There are two main trends in atomic radius. One atomic radius trend appears as you move across the periodic table from left to right (doing so within a period), and the other trend appears as you move from the periodic table’s top-down (moving within a group). To help you comprehend and visualise each atomic radius trend, the periodic table below includes arrows that show how atomic radii change.
1. Atomic Radius Trend 1: Atomic Radii Decrease From Left to Right Across a Period
The first atomic radius periodic trend is that as you move from left to right across a period, atomic size decreases. Each additional electron is added to the same shell within a group of elements. A new proton is also added to the nucleus when an electron is added, increasing the nuclear attraction and boosting the positive charge of the nucleus.
In other words, as protons are added, the nucleus gains a stronger positive charge, which in turn attracts the electrons more strongly and draws them in toward the nucleus of the atom. The radius of the atom decreases as the electrons are drawn inward toward the nucleus.
2. Atomic Radius Trend 2: Atomic Radii Increase as You Move Down a Group
Atomic radii rise as you descend in a group in the periodic table, which is the second atomic radius periodic trend. The atom gains an additional electron shell for every group down. The atomic radius grows as each new shell is positioned farther from the atom’s nucleus.
Contrary to popular belief, electron shielding keeps the valence electrons from the nucleus (those in the outermost shell). The electron shielding effect, which occurs when an atom has more than one electron shell, reduces the attraction of the outer electrons to the atom’s nucleus. As a result, electron shielding prevents the valence electrons from getting very close to the atom’s centre, increasing the atom’s radius.
Summary
Atomic radius is characterised by two major trends. The first periodic trend in atomic radii is the increase in atomic radius with decreasing group size. Electron shielding is the cause of this. When a new shell is added, the atomic radius grows as a result of the new electrons’ increased distance from the atom’s nucleus. More protons give an atom a stronger positive charge, which attracts electrons more strongly and pulls them toward the nucleus, shrinking the size of the atom. According to the second atomic radius periodic trend, atomic size decreases from left to right across the period.
Frequently Asked Questions
1. The atomic radius of which of the atoms-Arsenic or Selenium, is the largest?
Ans. Arsenic has a larger atomic radius than Selenium. The reason for this is that the extra protons increase the positive charge in the nucleus, which pulls the electrons closer together, reducing the radius. Arsenic along with Selenium is on the bottom row of the possibilities, but Arsenic is to the left. As a result, its atomic radius is the greatest.
2. What is the atomic radius of F and Ne in Angstrom?
Ans. The atomic radius of F and Ne in Angstrom is 0.72, 1.60. Noble gas elements quoted radii are “van der Waals radii,” which are 40 percent larger than their true atomic radii. As a result, the atomic radius of neon must be substantially larger than that of F.
3. Is it the size of Ne or \({\bf{N}}{{\bf{a}}^ + }\) that is smaller? Why?
Ans. \(N{a^ + }\) = proton number = 10
Ne = proton number = 10
Both are isoelectronic species, meaning they have the same number of electrons and shells (10 electrons). The size will be determined by the number of protons and nuclear charge. Because the sodium ion has 11 protons, the higher the nuclear charge, the stronger the nucleus’ affinity to valence shell electrons, and the size shrinks. The size of \(N{a^ + }\) is smaller than that of Ne.