Introduction
“Titration, also known as titrimetry, is a common quantitative chemical analysis method used in laboratories to determine the unknown concentration of an identified analyte. Because volume measurements are important in titration, it is also referred to as volumetric analysis. As a standard solution, a reagent known as the titrant or titrator is prepared. To determine the concentration, a known concentration and volume of the titrant reacts with a solution of the analyte or titrand. The volume of titrant reacted is referred to as the titration volume. Titrations come in a variety of forms, each with its own set of procedures and objectives. Acid-base titrations and redox titrations are the two most common types of qualitative titration.”
What do you understand by the term Acid Base Titration?
“An acid-base titration is a technique used to experiment with and learn about a solution containing an acid or a base. A base (alkali) is titrated with an acid, and an acid is titrated with a base (alkali). In Titration, the endpoint is determined by the use of an indicator. Acid-base titrations are in use to calculate the amount of a known acidic or basic substance through acid-base reactions. Titration refers to determining the concentration or rank of a solution in relation to water with a pH of 7. A standard solution is added using a device known as a burette. Titration is the process of adding a standard solution until the reaction is complete. It is understood that the substance to be determined is titrated.”
Types of Acid Base Titration
- Strong Acid and Strong Base-When a strong acid and a strong base react, exothermic reactions always occur. For example, a reaction with Hydrochloric Acid (HCl) and Sodium Hydroxide (NaOH).
\[{\bf{NaOH}}{\rm{ }} + {\rm{ }}{\bf{HCl}}{\rm{ }} \to {\rm{ }}{\bf{NaCl}} + {{\bf{H}}_2}{\bf{O}}\]
- Strong Acid and Weak Base-Example of such types are when Hydrochloric Acid (Strong Base) reacts with Ammonia (Weak Base)
\[{\bf{N}}{{\bf{H}}_3} + {\rm{ }}{\bf{HCl}}{\rm{ }} \to {\rm{ }}{\bf{N}}{{\bf{H}}_4}{\bf{Cl}}\]
- Weak Acid and Strong Base-Example of such types are when Ethanoic acid (Weak Acid) and Sodium Hydroxide (Strong Base) react
\[{\bf{C}}{{\bf{H}}_3}{\bf{COOH}}{\rm{ }} + {\rm{ }}{\bf{NaOH}}{\rm{ }} \to {\rm{ }}{\bf{C}}{{\bf{H}}_3}{\bf{COONa}}{\rm{ }} + {\rm{ }}{{\bf{H}}_2}{\bf{O}}\]
- Weak Acid and Weak Base-Example of such types are when the reaction between Ethanoic Acid (Weak Acid) and Ammonia (Weak Base) occurs.
\[{\bf{C}}{{\bf{H}}_3}{\bf{COOH}}{\rm{ }} + {\rm{ }}{\bf{N}}{{\bf{H}}_3} \to {\rm{ }}{\bf{C}}{{\bf{H}}_3}{\bf{COON}}{{\bf{H}}_4} + {\rm{ }}{{\bf{H}}_2}{\bf{O}}\]
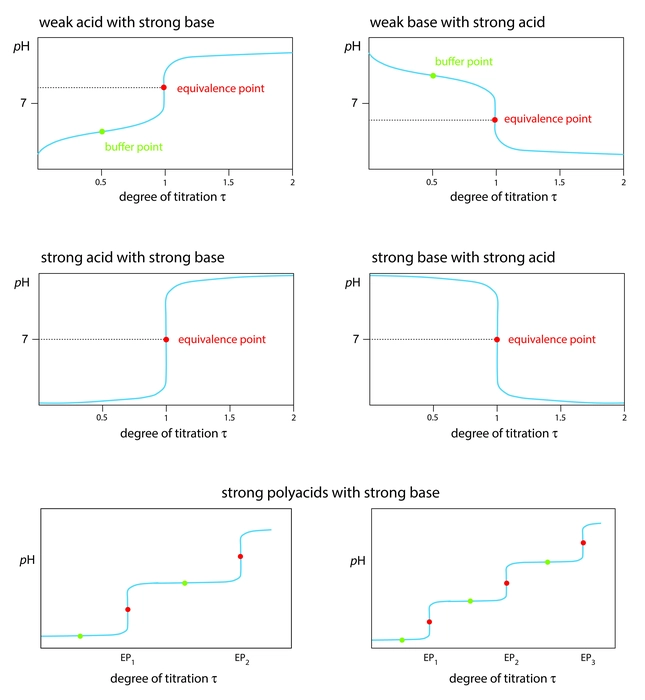
What is a Titration Curve
A titration curve is a plot of the analyte solution’s pH versus the amount of titrant added as the titration progresses.
- Strong Acid and Strong Base– As the titration begins with a strong acid, the pH of the solution is extremely low (around 1) when no base is added. The pH gradually rises as the base is gradually added. The equivalence point is the point at which all acids and bases have been neutralized. On the plot, this is indicated by a sharp increase or jump in pH. As we continue to add a base, the pH of the solution rises dramatically.
- Strong Acid and Weak Base- The solution’s initial pH indicates a weakly acidic solution. The titrant is a strong base, as indicated by the high final pH. The equivalence point is at a pH greater than 7.
- Weak Acid and Strong Base-At the start of the titration, the pH of the solution is approximately that of the weak acid in water. All the weak acid is neutralized and converted to their conjugate base at the equivalence point. The pH at the equivalence point, however, does not equal 7. This is because a conjugate base is produced during the titration. The resulting solution is somewhat simplistic.
- Weak Acid and Weak Base-The pH change around the equivalence point decreases significantly as the acid or base being titrated weakens (its \({\rm{p}}{{\rm{K}}_{\rm{a}}}{\rm{or p}}{{\rm{K}}_{\rm{b}}}\) increases). The curve becomes so shallow with very dilute solutions that it can no longer be used to determine the equivalence point.
What are the uses of Acid-Base Titration?
- Acid-base titrations are generally preferred for determining an analyte’s unknown acid or base concentration.
- Acid-base titrations are a type of chemical analysis that is measurable.
- Titrations of acids and bases have the potential to be used in pharmaceutical applications.
- Titrations of acids and bases can be used in environmental analysis.
Summary
A titration is a method of carrying out a chemical reaction between two solutions by controlling the addition of one solution (the titrant) from a buret to the other, allowing measurements to be taken throughout the reaction. A titration is useful for measuring the pH of an acid-base reaction at various points throughout the reaction.
Frequently Asked Questions
1. What is Titrant?
Ans. The titrant is a chemical solution of a known concentration that is added in titration.
2. What is an Equivalence point?
Ans. The equivalence factor in titration is the factor at which simply sufficient titrant is brought to absolutely neutralize the analyte answer. The answer best incorporates salt and water on the equivalence factor in an acid-base titration,
Moles of acids=moles of bases
3. What is Buffer solution?
Ans. The buffer answer is described as an answer that doesn’t alternate in Hydrogen ion attention whilst a small quantity of acid or base is brought to it.