Introduction
Old chemical bonds are cleaved in a chemical reaction and form new bonds. Any chemical equation should be balanced properly. It means the number of each atom should be the same on both the reactant and product sides. It is based on two rules; the ‘law of conservation of masses’ and the ‘law of constant proportions’. If a reaction is considered to be \(X + Y{\rm{ }} \to {\rm{ }}Z + P\), then X, Y are called reactants, and Z, P are called products of this reaction.
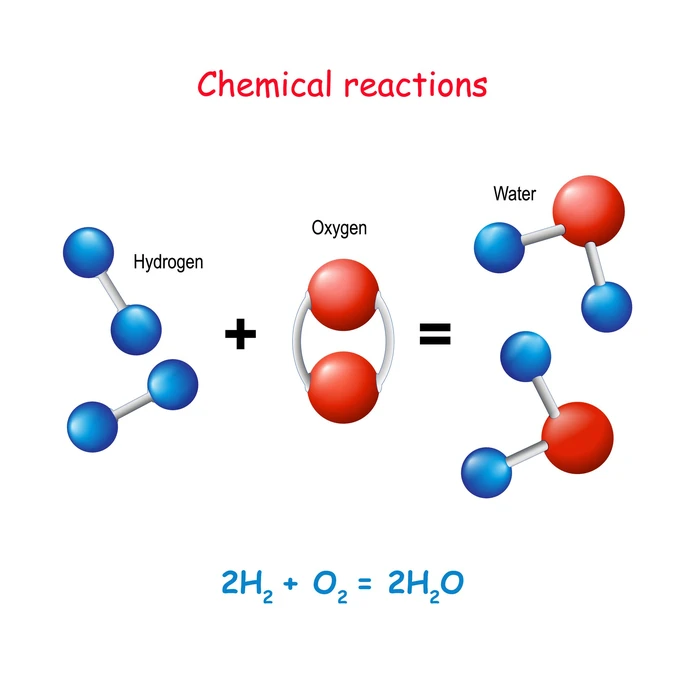
Define the law of conservation of mass.
It is stated in this law: “The mass in an isolated system can neither be created nor be destroyed but can be transformed from one form to another”. So the number of each type of atom in a chemical equation is always the same on both sides of the equation.
Read More: Law of Conservation of Mass with Experimental
Define the law of constant proportions.
The law states that- “In a chemical substance, the elements are always present in definite proportions by mass”. In the \({H_2}O\) molecule, the molar mass of two H atoms is 2 gm/mole and the molar mass of one O atom is 16 gm/mole. So their ratio of mass is 2:16=1:8. This ratio in \({H_2}O\) is always constant.
What is a balanced chemical equation?
According to the two laws of conservation of mass and conservation of definite proportions, a chemical equation must be properly balanced. It means that the number of all the atoms or molecules involved in a chemical reaction must be the same on both the reactant and product side. This is known as a balanced chemical equation.
Importance of coefficients and subscripts in balancing a chemical equation
Coefficients are numbers that help us to determine the number of each atom present in a balanced chemical equation. It can be changed necessarily.
Subscripts are the numbers that help to determine the chemical formula of any compound. The subscripts are always constant throughout a chemical equation.
\[{N_2} + {\rm{ }}3{\rm{ }}{H_2} \to {\rm{ }}2{\rm{ }}N{H_3}\]
Method of generating a balanced chemical equation-
Suppose we are trying to balance this unbalanced chemical equation.
\[C{H_4} + {O_2} \to {\rm{ }}C{O_2} + {H_2}O\]
These are the steps that are followed to make a balanced chemical equation.
- At first, the number of each atom on both sides is determined.
| Atoms present | Number of atoms on the reactants side | Number of atoms on the products side |
| C | 1 | 1 |
| O | 2 | 3 |
| H | 4 | 2 |
- Then coefficients of each atom are balanced properly. For this equation, at first, the coefficients of H are balanced. So now the chemical equation transforms into-
\[C{H_4} + {O_2} \to {\rm{ }}C{O_2} + 2{\rm{ }}{H_2}O\]
- Now the coefficient of O is balanced accordingly. So the new chemical equation is:
\[C{H_4} + 2{O_2} \to {\rm{ }}C{O_2} + 2{H_2}O\]
This is the balanced chemical equation: \(C{H_4} + 2{O_2} \to {\rm{ }}C{O_2} + 2{H_2}O\)
Balancing the chemical equation-
\[{C_3}{H_8} + {O_2} \to {\rm{ }}C{O_2} + {H_2}O\]
- At first, the number of each atom on both sides is determined.
| Atoms present | Number of atoms on the reactants side | Number of atoms on the products side |
| C | 3 | 1 |
| O | 2 | 3 |
| H | 8 | 2 |
- Now the coefficient of C is balanced on both sides. So the chemical equation changes to-
\[{C_3}{H_8} + {O_2} \to {\rm{ }}3C{O_2} + {H_2}O\]
After equating the coefficients of H, the new equation is:
\[{C_3}{H_8} + {O_2} \to {\rm{ }}3C{O_2} + 4{H_2}O\]
- Then the coefficients of O are balanced accordingly to form a balanced chemical equation.
\[{C_3}{H_8} + 5{O_2} \to {\rm{ }}3C{O_2} + 4{H_2}O\]
This is the balanced chemical equation: \({C_3}{H_8} + 5{O_2} \to {\rm{ }}3C{O_2} + 4{H_2}O\)
In this way, any chemical equation can be balanced.
Summary
According to the laws of conservation of mass and conservation of constant proportions, any chemical equation should be balanced properly. This is done by equating the coefficients of each atom involved in a chemical reaction. Balancing a chemical equation is extremely important in the field of chemistry. Based on the coefficients present before the molecules involved in a chemical equation, the yield of the products of that reaction can be determined.
Frequently Asked Questions
1. State the limitations of using chemical equations.
Ans: By any chemical equation we can’t understand the states(solid/liquid/gas) of the compounds involved. Again, the reversibility or irreversibility of any reaction can’t be determined by the chemical equation.
2. What are the different types of chemical equations?
Ans: Depending on the nature of reactants and products in a reaction, it may be classified into five types. They are combination reaction, single replacement reaction, decomposition reaction, combustion reaction, and double replacement reaction. Some reactions fall under two categories simultaneously.
3. What is the main reason behind a chemical reaction?
Ans: A chemical reaction can be described as a bond-breaking and bond-making process. It means all the old bonds are cleaved and new bonds are formed. The molecules which react in a chemical reaction are called reactants and the molecules produced in a reaction are called products.