Introduction
Something that has mass, takes up space, and can be sensed by our five senses is said to be matter. We can put it simply by saying that the things we see and feel around us matter. There are different states of matter. Because of the characteristics of the constituent particles and how they interact, each of these forms of matter has a unique feature. Atoms and molecules make up these particles. The basic elements of matter, atoms, are capable of independent existence. The neutron, proton, and electron subatomic particles that make up each atom determine the characteristics of the atoms.
Matter
The matter is a combination of two or more pure elements. The classification of the material into solids, liquids, and gases is based on its physical characteristics. Its classification into elements, compounds, and mixtures is based on its chemical characteristics. Our surroundings can be either geographical or man-made. Geographical surroundings are formed by nature and affect the social and economic climate, while man-made environments are those that are man-made.
All living and non-living things are called matter because they contain mass and take up space, all forms of life, including gases like oxygen and hydrogen, are referred to as matter. The DNA in our cells, the ground we are standing on, electrons revolving around a nucleus, or any other object is matter.
Types of Matter
The matter is divided into the three categories below based on its physical nature:
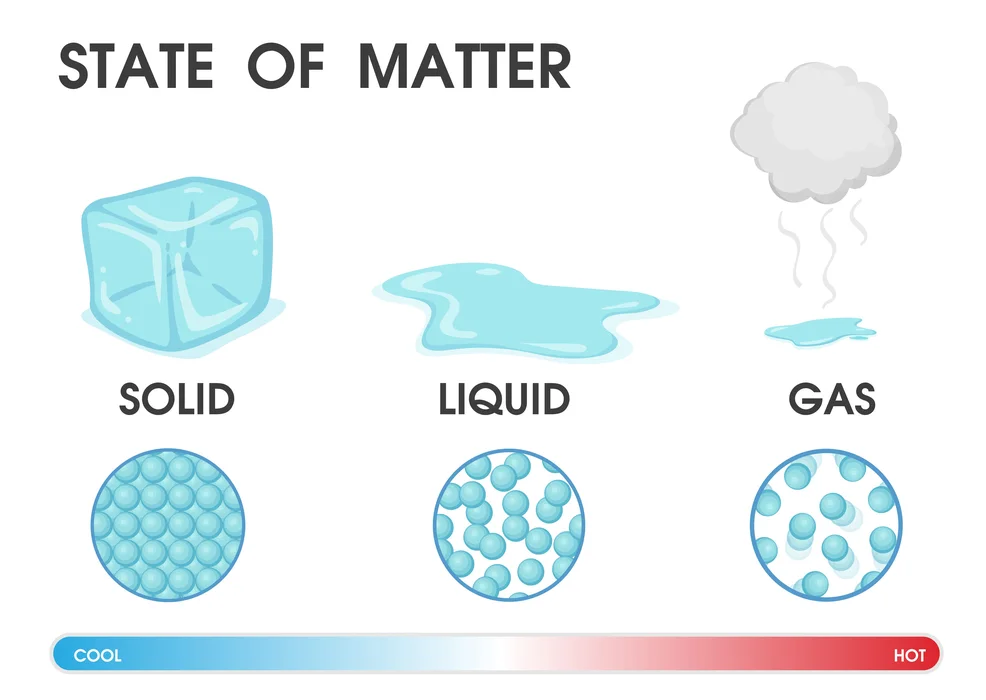
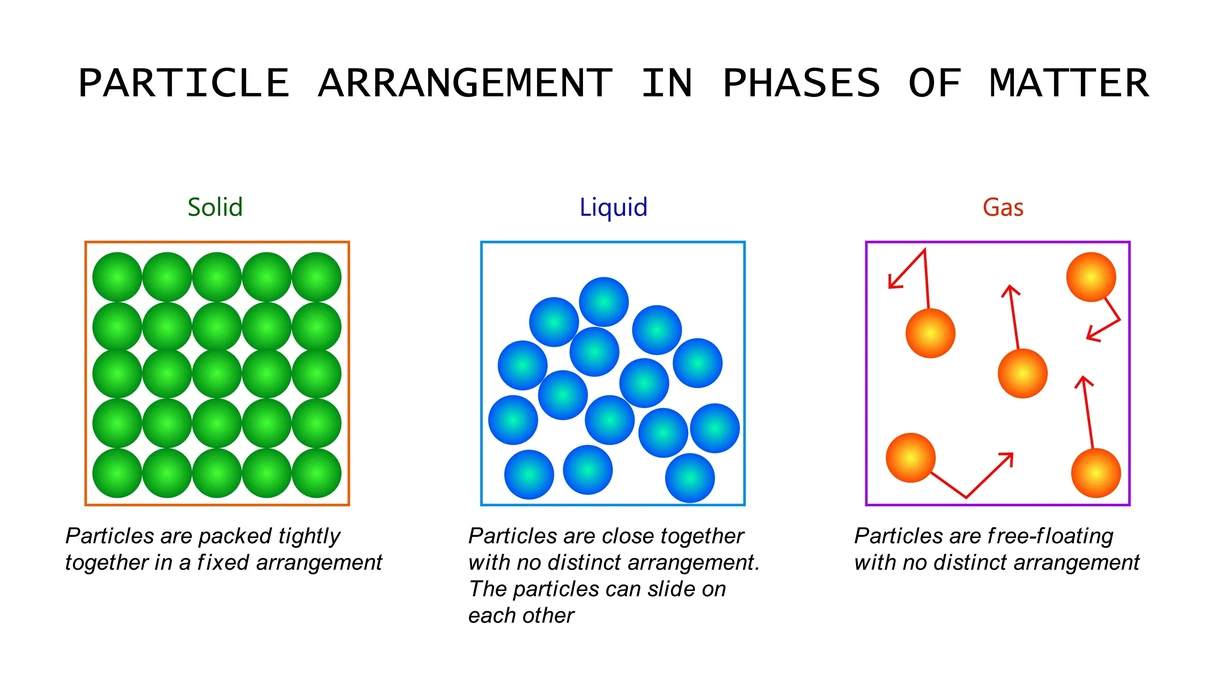
- Solids: Particles in solids are so closely packed and held in place by extremely strong intermolecular interactions that only vibratory motion is possible. They have a distinct volume and shape. Wood, iron, etc. are some examples.
- Liquids: Compared to solids, liquids have more freedom of movement due to the weak intermolecular interactions that allow for particle movement. Despite taking on the shape of the container they are poured into, they have specific volumes. Examples include milk, water, etc.
- Gases: These molecules move very freely and have a weak intermolecular interaction. The distance between them is also very large. They fill the container in which they are placed because they lack a set shape and a volume. Examples include hydrogen and methane.
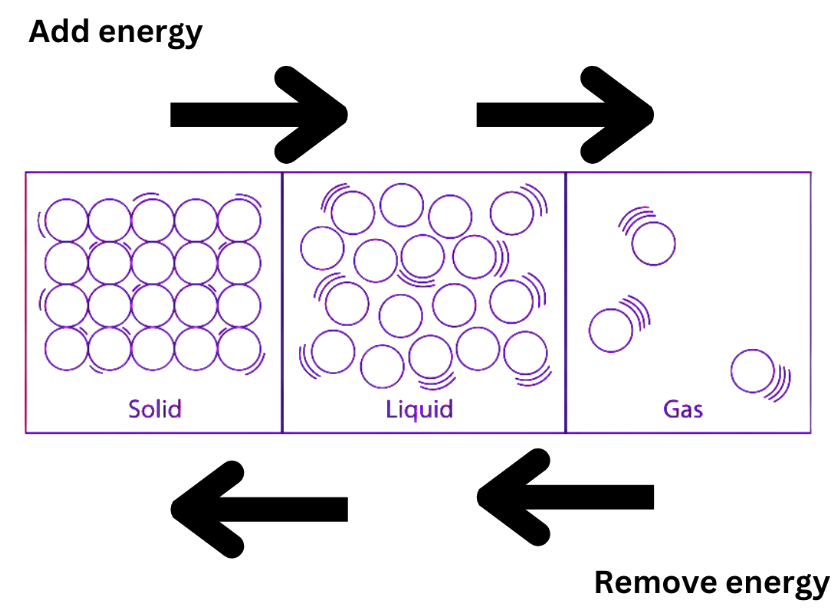
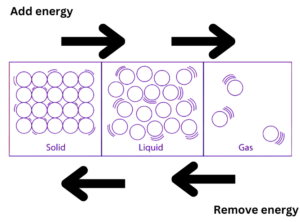
Applying pressure and changing the temperature can modify the nature of the three matter states mentioned above. There are particles in a matter that have kinetic energy; this energy rises with temperature. In solids, the distance between particles and kinetic energy is the smallest, whereas it is greatest in gases. The three types of matter that make up our environment are interchangeable through temperature changes. For instance, changing the temperature will cause ice to turn into water and back again.
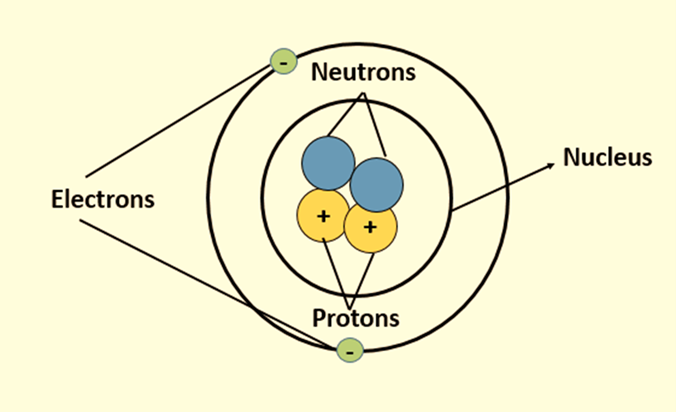
Subatomic Particles
Protons, neutrons, and electrons make up the primary units of matter, known as atoms. Protons have a positive charge, whereas electrons have a negative charge, making neutrons neutral particles with no charge. The nucleus of an atom is made up of neutrons and protons, and electrons revolve around this nucleus in their respective orbitals. The quantity and configurations of these subatomic particles greatly influence the stability and characteristics of the atom.
Summary
The Panch Tatva, or air, earth, fire, sky, and water, was the system used by our ancient Indian thinkers to categorize matter. There are billions of atoms in every gram of matter. The matter is everything that has mass and takes up space. Matter is composed of particles that are always moving and have different properties in each of the three states of matter. The particles of matter are very tiny and have space between them. The three types of matter that make up our environment are interchangeable through temperature changes.
Frequently Asked Questions
1. What features do matter particles have?
Ans: The characteristics of matter particles are given below:
a) The intermolecular space that particles have is one of their distinguishing characteristics.
b) Intermolecular force exists among particles.
c) Matter is made up of moving particles.
2. In comparison to solids, liquids typically have a lower density. You must have seen that ice floats on water, though. Why?
Ans: Although ice is a solid, due to its structure, it has a lesser density than water. Ice floats on water because its molecules form a cage-like structure with lots of empty spaces.
3. How can water stored in a matka (earthen pot) cool throughout the summer?
Ans: Since the clay pot has many pores and is porous, the water seeps out of them and evaporates on the pot’s surface, which has a cooling effect. This chills the pot, which in turn causes the water inside to cool.