Metals and Non-Metals
Introduction
The crust of the Earth is packed with a variety of abundant and inexhaustible minerals, as we have studied in our geographical class. These minerals are a blend of natural elements that are extracted and used for various things. Each element and mineral has unique qualities of its own that make it useful.
Metals
Metals are among the sorts of elements that make up the crust of the Earth. They are a combination of substances that are frequently hard, malleable, ductile, glossy, etc. Metals are also effective electrical conductors. They can be found in a free state (without any combination) or a mixed state in the Earth’s core (with a mixture of oxygen, rock, and dust).
Non-Metals
Non-metal elements are those that do not exhibit non-metallic properties. They are not malleable like metals and have different physical characteristics from metals. They are brittle and ductile as well. When compared to metals, they have a low density. Non-metals can be solid, liquid, and gas, and they are mostly bad conductors of electricity. Non-metals include things like oxygen, phosphorus, and sulphur.
How Do Metals and Non Metals React with Each Other?
- Metals react with non-metals by transferring electrons from metal atoms to non-metal atoms, resulting in the formation of ions.
- This process produces an ionic compound.
- Metal atoms transfer electrons to non-metal atoms.
- Metal atoms become positive ions, while non-metal atoms become negative ions.
- Example Sodium Chloride (NaCl)
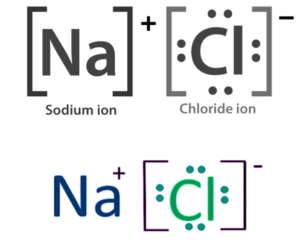
What Reactions Occur Between Metals?
Metals interact with one another by their degree of reactivity, or strength. The less reactive metal is displaced when a metal reacts more vigorously than the metal with which it is bonding. It could be solid, liquid, or molten.
In other words, metal A is more reactive than metal B if it displaces metal B. Galvanic corrosion results from the collision of two metals. Two distinct metals connected by an electrolyte are required for galvanic corrosion to occur. If this occurs, the corrosion process will be initiated by the electrolyte. More reactive metals corrode more frequently.
Salt solution of A + Metal B > Salt solution of A + Metal
\[Zn\left( s \right){\rm{ }} + {\rm{ }}CuS{O_4}\left( {aq} \right) \to {\rm{ }}ZnS{O_4}\left( {aq} \right){\rm{ }} + {\rm{ }}Cu\left( s \right)\]
Summary
The concept of metals, non-metals, and their properties are all included in this article. The characteristic of metals and non-metals differ, and each has a unique state of reactivity. Each element and mineral has distinct properties of its own that make it useful. A variety of organic elements are combined to form these minerals, which are then extracted and used for various purposes.
Frequently Asked Questions
1. What Characteristics do Non-Metals Generally have?
Ans. The characteristics of non-metals are as follows.
- A metal’s qualities depend on its size.
- They often have bad electrical conductivity. The main characteristic that sets them apart from metals is this one.
- Due to their greater electronegativity, non-metals have a higher potential of attracting more electrons.
- They retain their electrons and borrow from the metals due to their higher electronegativity.
- The states of non-metals include solid, liquid, and gas. They are brittle by nature in the solid state, giving rise to ductile and non-malleable states.
2. What Significant Function do Non-Metals Play in Our Lives?
Ans. Non-metals such as nitrogen and phosphorus are used in fertilizers to increase plant yield. Phosphorus is used to make matchsticks and fireworks. Chlorine, a non-metal, is used in the water purification process. Carbon, a non-metal, is used in the majority of fuels.
3. What Happens to Metals During Recycling?
Ans. The majority will be smelted into ingots, so they can be melted and processed at metal facilities across the nation. The recycling facilities’ rubbish to the grocery store shelves can be accessible within as little as six weeks.