Introduction
In today’s world, acid rain is a serious environmental issue. Midway through the 19th century, it was reported. As a result of excessive contaminants being removed from the atmosphere by both natural and man-made sources, this has occurred and therefore bringing down the pH of regular rainwater. There is no way to lessen the impact of natural sources, but there are various preventive steps that can be taken to lessen the impact of man-made sources. Since acid rain affects several ecosystems by lowering the pH of the material where it has been deposited, its prevention or mitigation is crucial.
What is acid rain?
Acid rain, a severe environmental issue, is caused by \(S{O_2}\) and \(N{O_x}\) emissions into the atmosphere. Acid rain is created when these substances interact with water vapour in the atmosphere. Normally, \(S{O_2}\) does not interact with water, but when it interacts with ozone, it changes into \(S{O_3}\), which then reacts with water to create sulphuric acid, resulting in acid rain. The environment is being threatened by acid rain. Water bodies and biological beings suffer greatly from acidification. According to reports, acidification causes a greater release of aluminium from rocks or soil and which eventually gathers or deposits on water bodies, posing a hazard to the creatures that drink the water.
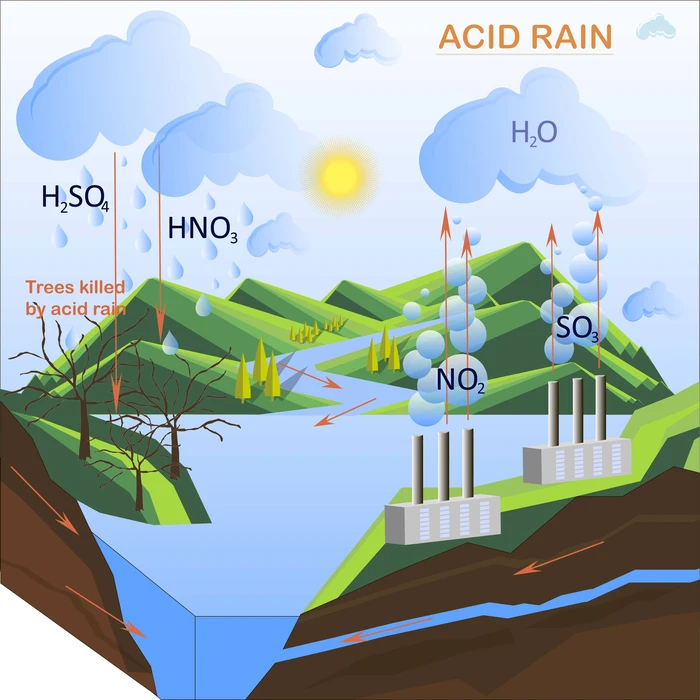
What are the causes of acid rain?
- The atmospheric release of \(S{O_2}\) and \(N{O_x}\) chemicals results in acid rain. These chemicals come from a variety of sources, including both natural processes and human activity.
- The natural sources include volcanic eruptions, sea spray, forest fires, etc. While man-made sources for sulphur dioxide include industrial combustion, coal burning from cars, oil refineries, home, and industrial boilers, automobiles, fertilizer plants, etc.
- Natural sources of \(N{O_x}\) include bacterial activity, volcanic eruption, lightning, forest fires, etc.
- When these substances are discharged in excess into the atmosphere, they react with the water that is already there to generate numerous acid molecules.
- They are sulphuric acid and nitric acid. These further contribute to acidification and bring about various environmental issues.
What are the effects of acid rain?
- Nitrates are deposited on the soil as a result of acid rain, which also raises nitrogen saturation levels. This may also result in the loss of other significant ions from the soil, including calcium and magnesium ions that are beneficial to trees and plants. Additionally, acid rain removes the protective layer of trees, weakening them.
- Since acids take aluminium from soil and deposit it in water bodies, acid rain also has an impact on surface waters by raising the concentration of aluminium in the water bodies. The aquatic species that live in the water bodies will ultimately be impacted by this.
- Since the increased concentration of sulphuric acid and nitric acid promotes the corrosion of metals and fading of paints, acid rain impacts have been documented in man-made sculptures.
- Due to the particle build-up, acid rain has an impact on people’s health who have asthma and emphysema.
- Visibility is hampered by acid rain, which can often appear as fog.
Real-life examples of acid rain effects
- Acid rain has caused the sculpture at Oakland University in Rochester, Michigan, to degrade.
- The US, Taiwan, Europe, Poland, and China’s southeast coast are some of the regions that are impacted.
- The glossy feature of the Taj Mahal is presently deteriorating as a result of acid rain.
- Due to acid rain, the Statue of Liberty corroded and turned green.
- Nearshore and coastal oceans are significantly acidified as a result of acid rain.
How to prevent acid rain?
Acid rain can be prevented only by reducing the emission of acid rain-causing particulates into the atmosphere. Only man-made sources can be reduced by performing several preventive methods. This can be done by,
a) Reducing the sulphur-containing fuels in automobiles or switching to alternative sources.
b) Scrubbers can be used for reducing the emission of \(S{O_2}\) into the atmosphere since they will attract sulphur particles.
c) For reducing the emission of \(N{O_x}\) catalytic converters can be used, thereby converting it into \({N_2}\) and \({O_2}\).
d) Liming water bodies can be done in reducing the impact of acid rain.
e) The effect on metals due to acid rain can be reduced by coating them with corresponding materials.
Summary
Acid rain, a severe environmental issue, is caused by the atmosphere’s \(S{O_2}\) and \(N{O_x}\) emissions. The atmospheric release of \(S{O_2}\) and \(N{O_x}\) chemicals results in acid rain. Nitrates are deposited on the soil as a result of acid rain, which also raises nitrogen saturation levels. The glossy feature of the Taj Mahal is presently deteriorating as a result of acid rain. The effect on metals due to acid rain can be reduced by coating them with corresponding materials.
Frequently Asked Questions
1. Write a note on the facts about acid rain.
Ans. Numerous substances, such as precipitation, snow, fog, and microscopic dry particles that fall on the surface of the planet, can carry acid rain to its surface. Additionally, regular precipitation has a pH of 5.6, whereas acid rain has a pH of roughly 4.6, which is a bigger difference and makes its effect more sensitive.
2. Can acid rain cause skin burns?
Ans. Since acid has a pH of 4.6, acid rain is not sufficiently acidic to cause skin burns. Additionally, it has no direct impact on humans. Patients with asthma and emphysema will experience issues if they come into contact with sulphur particles in the air.
3. Where does acid rain occur most often?
Ans. Acid rain is a widespread problem in places like Eastern Europe, and the US, and is now spreading to parts of India and China.