Introduction
The scientist, Antonio Lavoisier, introduced the law of conservation of mass in the year 1789. According to him, mass can’t be destroyed or generated. But mass can be transformed from one form to another. In our daily life, we also utilize this law. For example, in the burning process of wood, the total masses of the products (gases, ashes, soot) and the masses of the reactants (charcoal and oxygen)remain the same.
Define “The Law of Conservation Of Mass”:
This law states: “matter cannot be created or destroyed in a chemical reaction”. That means the total masses of reactants or products should be the same after a reaction.
Application of the law of conservation of mass in different reactions:
1. During phase transition: During the transformation of any substance from its solid to liquid and then to vapour, the total mass of the substance remains constant. In the physical change of ice to water and then water to vapour, the mass of water in its three states remains the same.
Ice ⇌ water ⇌ vapour
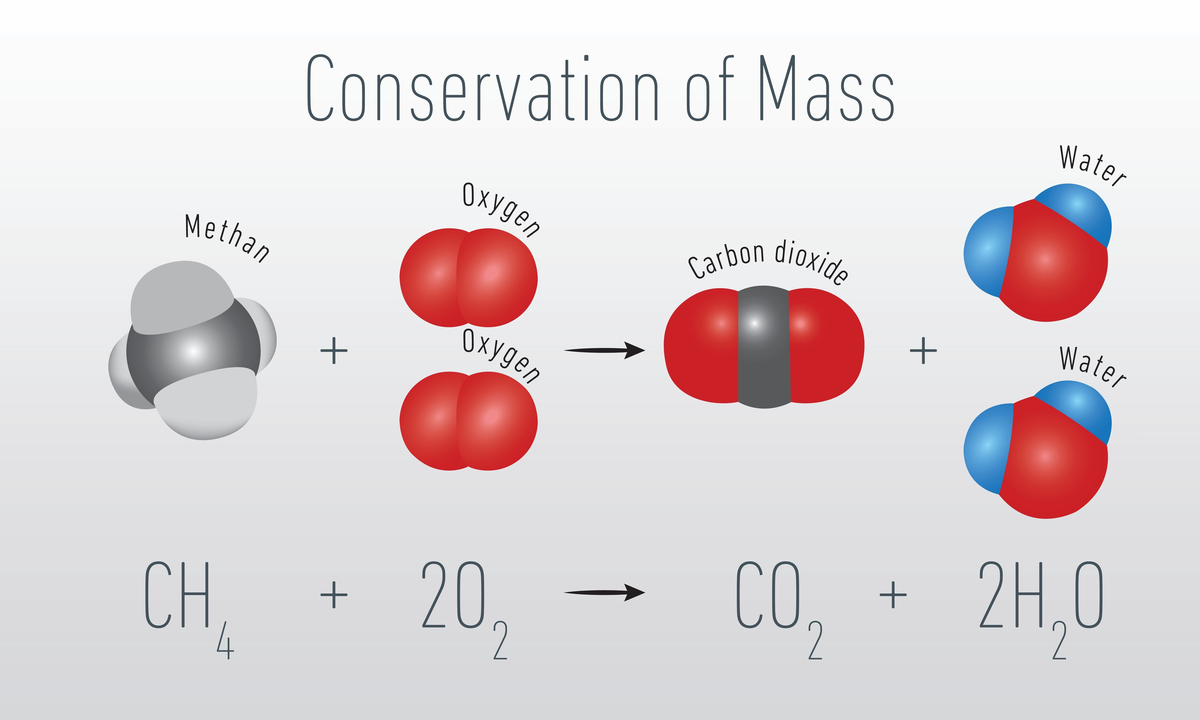
2. During chemical reaction: Total masses of the reactants and the products remain the same after a successful chemical reaction. Like, in the combustion reaction of methane, carbon dioxide \(C{O_2}\) and water \({H_2}O\) are produced. The total masses of reactants (\(C{H_4}\)and \({O_2}\)) remain the same as the masses of products (\(C{O_2}\) and \({H_2}O\)).
\[C{H_4} + 2{O_2} \to C{O_2} + 2{H_2}O\]
3. During rearrangement reaction: Calcium carbonate produces calcium oxide and carbon dioxide on heating. The masses of reactants (calcium carbonate) and the products (calcium oxide and carbon dioxide)remain the same.
\[CaC{O_3} \to {\rm{ }}CaO{\rm{ }} + {\rm{ }}C{O_2}\]
Methods to examine the law of conservation of mass:
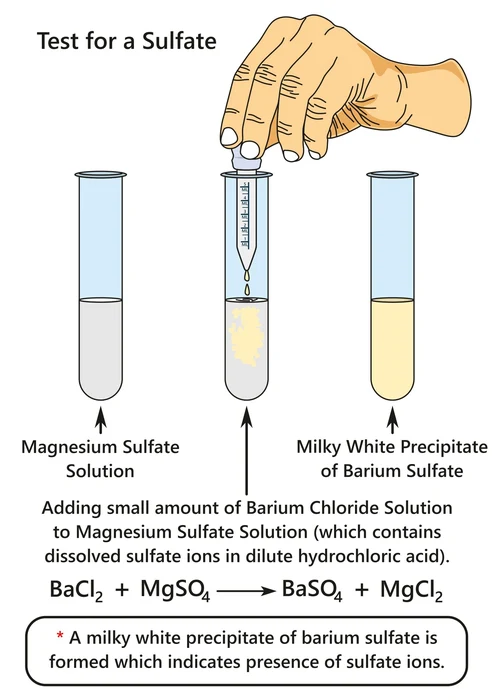
1. In the reaction of Barium chloride and magnesium sulfate: The law of conservation of mass can be proved by the following reaction. For this, some steps need to be followed.
- First, a particular amount of Barium chloride (\(BaC{l_2}.2{H_2}O\)) is weighed. Then some amount of distilled water is mixed with it. And this mixture is named A.
- Then some definite amount of magnesium sulfate (\(MgS{O_4}\)) is mixed with the same amount of water as A. This mixture is indicated as B.
- Then another empty beaker(C) is taken and weighed. The whole solution of A and B is poured into beaker C.
- A white precipitate of \(BaS{O_4}\) is formed in beaker C. Then the weight of beaker C is taken again.
- Now the weight of empty beaker C is subtracted from the beaker C, containing products.
- Comparing the masses of A, B, and C, we get that the total masses of the solutions A and B match with the product formed in beaker C.
In this way, the conservation of mass can be proved.
\[BaC{l_2} + {\rm{ }}MgS{O_4} \to {\rm{ }}BaS{O_4} + {\rm{ }}MgC{l_2}\]
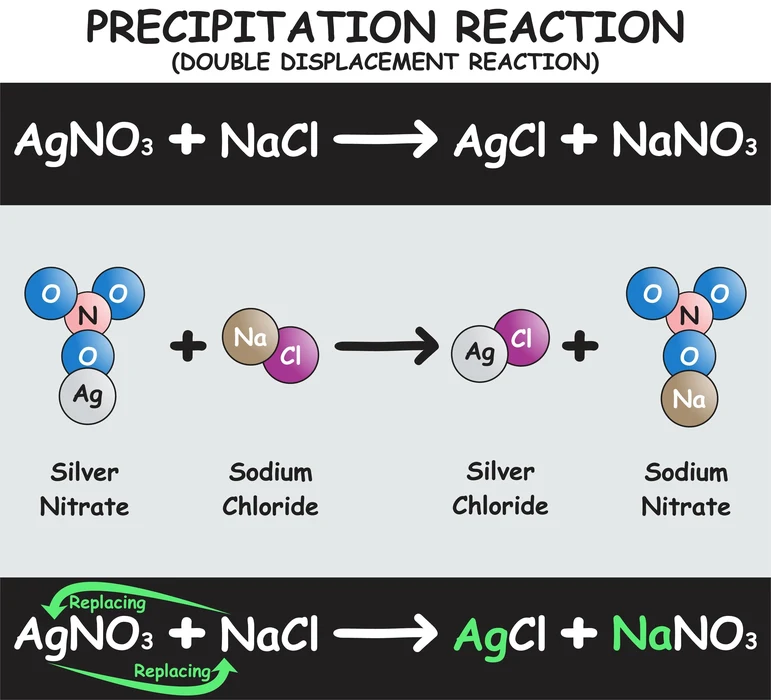
2. In the reaction of silver nitrate and sodium chloride: This law can be proved by the following reaction of silver nitrate and sodium chloride. For this, the following steps need to be followed.
- Silver nitrate and sodium chloride solutions are made separately. Sodium chloride solution is taken in a conical flask and silver nitrate is taken in an ignition tube.
- Then the ignition tube is suspended into the flask and the flask is fitted with a cork.
- Now the mass of the conical flask is recorded, and then the conical flask is tilted for the reaction of silver nitrate and sodium chloride.
- Now, the product \(AgCl\) is precipitated as a solid. At this stage, the mass of the conical flask is recorded again.
- It is found that the masses of reactants and products are the same.
\[NaCl{\rm{ }} + {\rm{ }}AgN{O_3} \to {\rm{ }}AgCl{\rm{ }} + {\rm{ }}NaN{O_3}\]
Summary
The law of conservation of mass tells us that the mass of reactants and products is always the same after a reaction. That is, the total mass is always conserved in different types of chemical reactions as well as in physical changes. This law can be proved by some reactions in the laboratory. This law is very essential as the unknown mass of any reactant or product can be determined. From this law, any chemical reaction can be balanced easily.
Frequently Asked Questions
1. What are the drawbacks of the law of conservation of mass?
Ans: This law is valid for only chemical reactions and physical changes. But does not apply to nuclear reactions. During a nuclear reaction, heat is produced. That means some mass is converted to heat. So the mass is not conserved in the nuclear reaction.
2. Is there any difference between the conservation of mass and the conservation of energy?
Ans: Conservation of mass and energy were commonly considered to be separate concepts. However, special relativity demonstrates that mass and energy are linked by the formula \(E{\rm{ }} = {\rm{ }}m{c^2}\), and science currently holds the belief that the sum of mass and energy is conserved.
3. Which equation corresponds with the principle of mass conservation?
Ans: The law of mass conservation can be expressed by a balanced chemical equation. In a balanced chemical equation, the number of each element involved in a reaction is always the same on the reactants and products side.