Introduction
John Dalton, an English philosopher and scientist, established the atomic notion in 1808 as part of his investigation of the molecular structure of matter. It asserted that everything is made up of atoms, which are supposedly tiny autonomous creatures. According to Dalton’s atomic theory, the constituent parts of these substances are atoms that cannot be broken down into smaller parts. These other elements’ atoms range in size and mass, whereas those of a particular element are nearly uniform.

John Dalton
Dalton’s Atomic Model
Indeed, matter studies have been among the most fruitful areas for scientists. Both scientists and philosophers have spent years trying to make the world easier to grasp. The properties, structure, and other characteristics of matter’s fundamental particles have long piqued the interest of scientists. Hence, several atomic theories have emerged. The idea that matter comprises smaller units, or “particles,” is credited to Democritus. Such tiny things have been given the Greek word for “indivisible” and “atom” as their name. Democritus’ Atomic Theory rests on this principle. As a result, technological infrastructure needed to be improved, and scholars knew very little about this concept then. John Dalton, a scholar from around 2,000 years ago, displayed their attempts to simplify the situation. John Dalton published his now-famous atomic theory in 1808. This hypothesis was first given by John in a paper he named “A New Chemical Philosophy,” and at the time, it was novel. Dalton’s theory revolved around two laws:
- Law of Conservation of Mass: Antoine Laurent Lavoisier developed the principle of mass conservation in 1789. Although the creation and destruction of matter are prohibited by law, matter can transform into another form within a closed system. Scientists use this rule to preserve linear equations.
- Law of Constant Composition: The rule of constant composition states that a pure material always has the same number of elements. For example, the sodium and chlorine content of NaCl, the chemical formula for table salt, remains constant regardless of how much salt we manufacture.
Postulates of Dalton’s Atomic Theory
Following are the postulates of Dalton’s Atomic Theory
- All matter is composed of tiny atoms and interconnected particles.
- There is no difference in mass, size, or any other physical property between iotas of different substances. Yet, due to their different characteristics, the mass and size of particles composed of different elements can vary widely.
- It was impossible to create or destroy an atom. Yet, iotas can’t be broken up into smaller pieces.
- Atoms of various elements mix in fixed, whole-number proportions to produce compounds.
- In compound reactions, atoms might undergo transformations, form bonds, and break free.
Merits of Dalton’s Atomic theory
Some of the key advantages of the theory are:
- It provides a basic idea for telling the difference between elements and compounds.
- Several proportional rules, mass conservation, and stable proportions are all upheld.
Limitations of Dalton’s Atomic Theory
Some of the key advantages of the theory are:
- Such an atom has been repeatedly proven to be divisible. Neutrons, protons, and electrons are the three possible constituents of an atom. Remember, though, that the smallest thing that may participate in a chemical reaction is one atom.
- According to Dalton’s atomic theory, all atoms of a given element have the same mass, size, and chemical and physical properties. The density and mass of atoms are both visible differences across elements. Isotopes are different varieties of an element that have different masses. The mass numbers of the two stable isotopes of Cl are 35 and 37, respectively.
- In addition, atoms of different elements have different sizes, masses, and other chemical and physical properties. That’s not always the case, though. For example, the atomic mass of an Ar or Ca atom is 40 amu. Atoms with the same mass number form an isobar.
- According to Dalton’s atomic theory, chemical compounds are generated when different constituents mix in simple whole-number proportions. Yet, this might not be the case with more complicated chemical compounds.
- In Dalton’s atomic theory, allotropes are not accounted for. The Dalton atomic hypothesis can no longer explain the observed differences in properties among charcoal, graphite, and diamond.
- According to Dalton’s atomic theory, the atom is the smallest unit capable of participating in a chemical reaction. Several of the underlying assumptions of this theory remain valid in modern chemistry. The proposed model of atomic structure represents a significant advance in chemical theory. It’s what modern atomic theories and quantum mechanics are built on.
Influences on Modern Atomic Theory
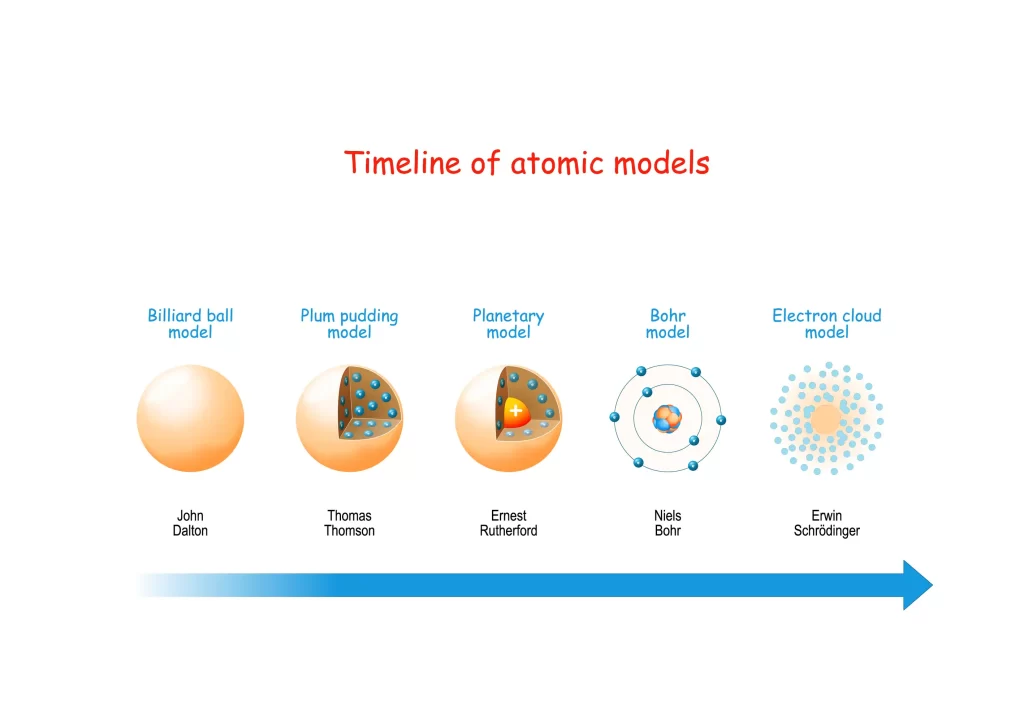
Many advances in modern atomic theory may be traced back to Dalton’s atomic theory. Dalton’s method was not only innovative for its day, but it also served as a foundation for subsequent chemists. Ernest Rutherford, Chadwick, Niels Bohr, JJ Thompson, and others after Dalton all made important contributions to the development of atomic theory. Eventually, JJ Thompson identified electrons, and Rutherford honed the hypothesis to pinpoint the nucleus. The atomic model as we know it today is thus a product of both Niels Bohr’s model and the Quantum mechanical model. Although the field of atomic theory has progressed much over the past two centuries, much of Dalton’s original framework remains.
Summary
Our analysis leads us to the conclusion that this concept represents John Dalton’s atomic model from 1808. According to Dalton, atoms are the fundamental building blocks of all matter. It also suggests that the different masses of atoms belonging to different elements are unaffected by chemical reactions. They wondered what the fundamental particles were like, how they were put together, and what properties they had. This led to the growth of several atomic theories. It would appear that the smallest unit of a substance that may take part in a chemical reaction is the atom. Even within the same element, atoms may have different masses. Dalton’s postulates for the atomic theory do not include this. This is a flaw in some theoretical frameworks.
Frequently Asked Questions
1. How does the presence of subatomic particles lead to the failure of Dalton’s atomic theory?
Thomson’s discoveries on subatomic particles were groundbreaking. This proved Dalton wrong when he claimed such atoms are the smallest possible material component. Thomson concluded from his findings that electrons are fundamental building blocks of atoms.
2. Which is the latest atomic model?
The electron cloud model is one of the most sophisticated and well-known current atomic models. Although it retains the nucleus concept from Bohr and Rutherford’s models, it provides a new description of electron velocity around the nucleus.
3. What gas makes up the cathode ray tube?
Hydrogen gas, the lightest gas (perhaps the lightest element) on ionisation, yields the highest charge value to the mass ratio (e / m ratio = \(1.76 \times {10^{11}}\) coulombs per kg), hence it is often used in cathode tube experiments.