Introduction
Lemon juice, soap, milk, detergents, and other frequently used items in daily life are all made of acids and bases. In addition to these sustainable acids and bases, a large variety of chemical or mineral acids or bases are also used. Knowing the fundamental characteristics of acids and bases is essential to comprehend the concepts of acids and bases. Water can be combined with acids and bases to produce two different aqueous solutions. Any material that dissolves in water releases free \({H^ + }\) ions to produce hydronium (\({H_3}{O^ + }\)), which is referred to as an acid and releases hydroxyl ions (\(O{H^ – }\)), which is referred to as a base. This acid and base will neutralize each other when combined.
Definition-Acids and Bases
Acids: Chemical substances or compounds that can donate a proton (\({H^ + }\)) or accept electron pairs are known as acids.
Examples include hydrochloric acid (HCl) and sulphuric acid (\({H_2}S{O_4}\)).
Bases are substances or ions that can take a proton or donate a pair of electrons.
Examples include potassium hydroxide (KOH) and sodium hydroxide (NaOH).
Theories of Acids and Bases
Arrhenius theory
1. According to Arrhenius, an acid is any chemical that increases the concentration of protons (\({H^ + }\)) in a solution. For Example, the (\({H^ + }\)) and (\(C{l^ – }\)) ions are created when the acid HCl (which is a base) dissolves in water.
2. Bases are compounds that increase the number of hydroxide ions (\(O{H^ – }\)) in solutions. Take NaOH as an example, which dissolves in water to produce the ions \(N{a^ + }\) and \(O{H^ – }\). Consequently, increasing the \(O{H^ – }\) ion concentration.
Brønsted Lowry theory
This theory states that bases are proton (\({H^ + }\)) acceptors and form a conjugate acid, whereas acids are proton (\({H^ + }\)) donors and form conjugate bases.
For example, hydrochloric acid (HCl), which is a Brønsted-Lowry acid, gives its proton to water when it is mixed with a base (\({H_2}O\)). Water is referred to be the Brønsted-Lowry base when it receives a proton.
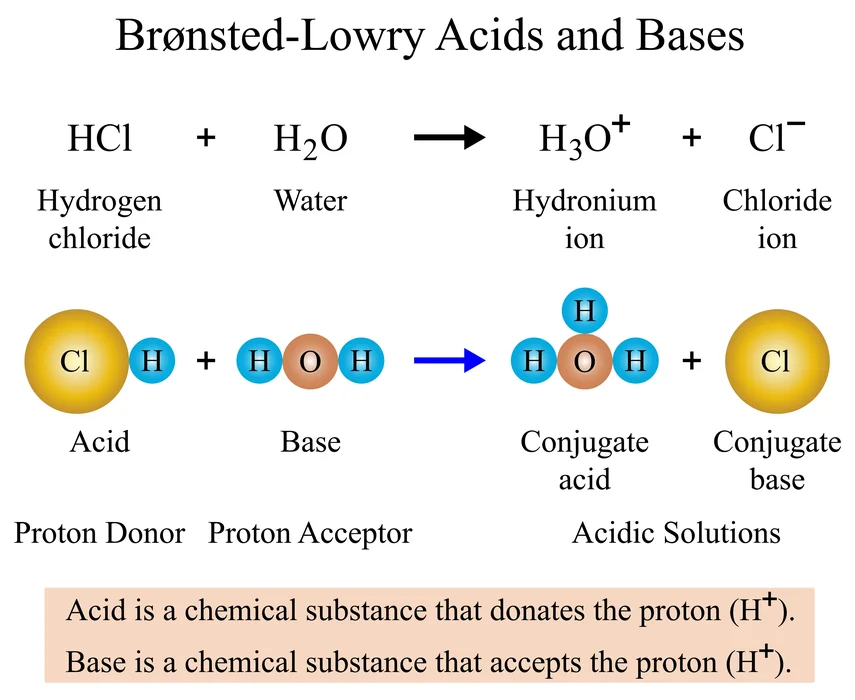
Acids and bases in conjugates
A conjugate base has one more negative (-) charge and one less H-atom than the acid that generated it, whereas a conjugate acid has one more positive (+) charge and one more H-atom than the base that formed it.
As an illustration, in this reaction, a base (\(N{H_3}\)) and an acid (HCl) combine to generate a conjugate acid (\(N{H^{4 + }}\)) and a conjugate base (\(C{l^ – }\)).
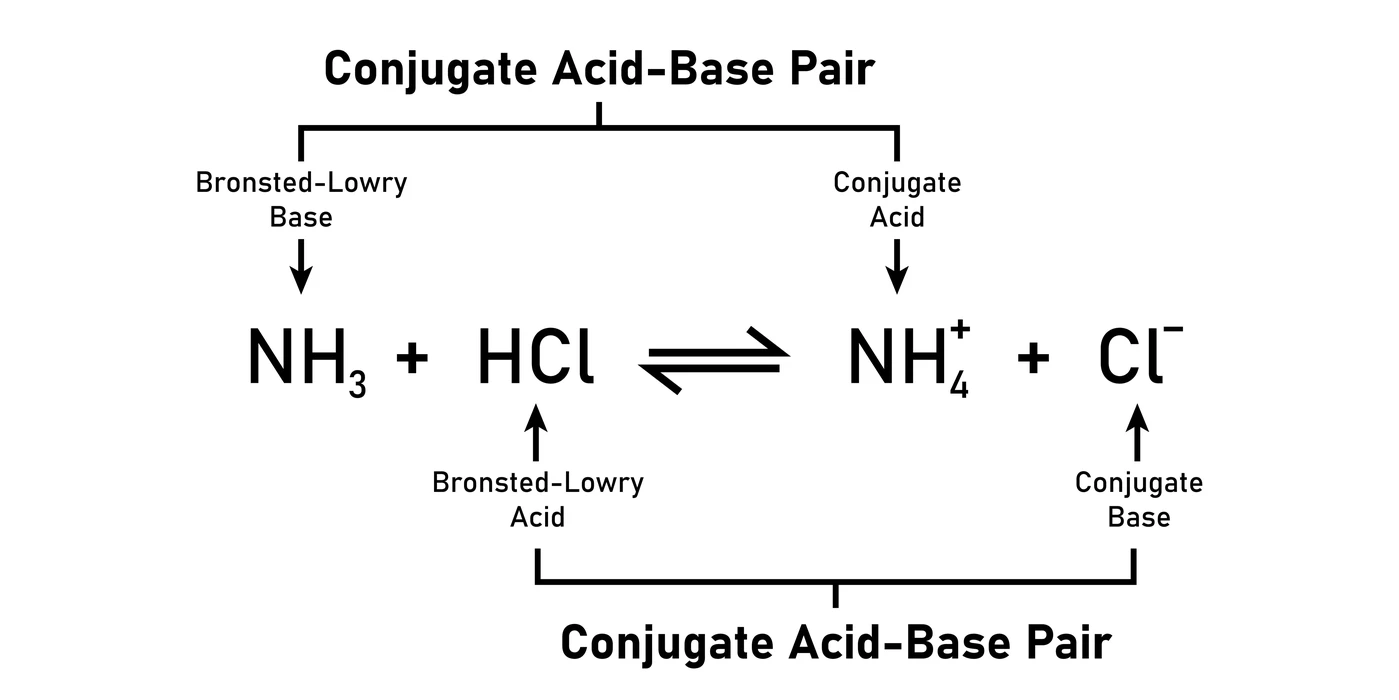
Lewis Concept of Acids and Bases
Lewis bases or acids are terms used to describe substances that take electron pairs, whereas Lewis bases or acids are terms used to describe substances that contribute to electron pairs. Thus, the acceptance or donation of electrons determines the acidity or basicity of a substance.
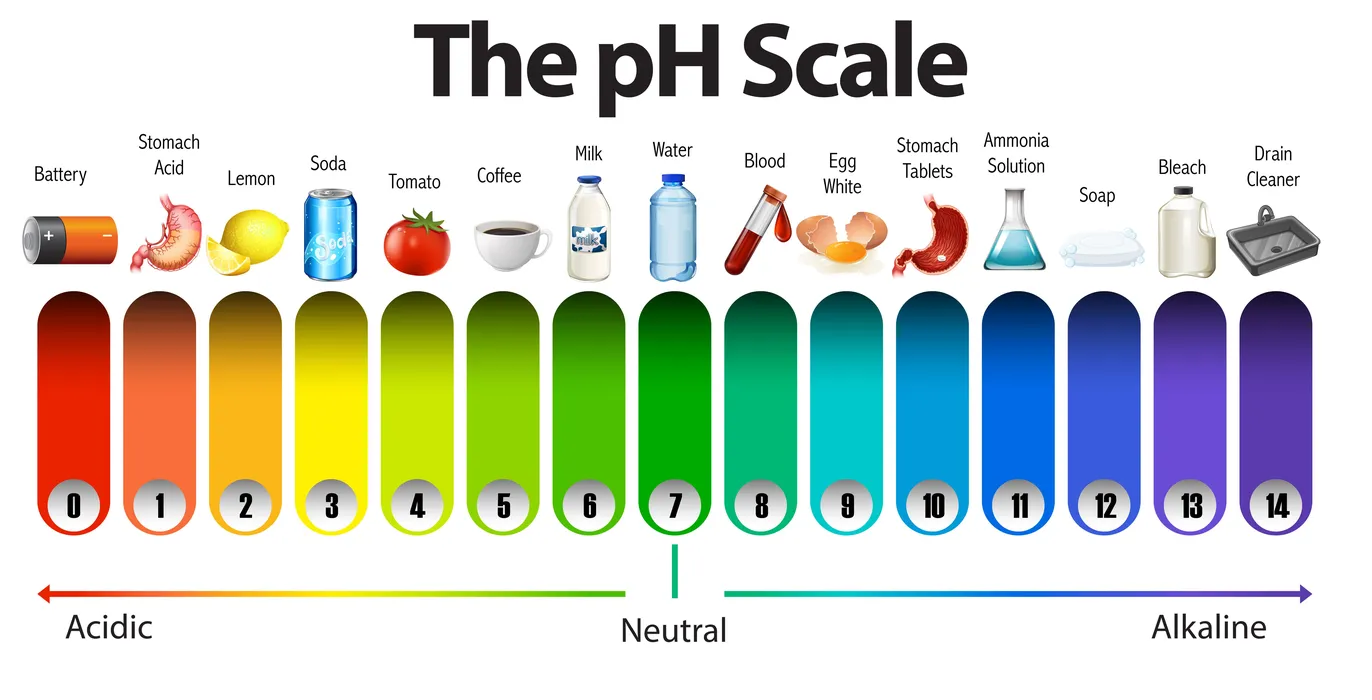
Acids and bases pH values
The word pH, which stands for potential hydrogen, helps scientists evaluate whether a solution is acidic or basic based on how many hydrogen ions are present in it. It is essentially a pH scale or negative logarithmic scale that ranges from 0 to 14 and quantifies the molar concentration of hydrogen ions from 1 to 14. It has no units for measurement.
The equation for calculating pH is pH =-log [\({H^ + }\)]
When a substance’s pH value is below 7, it is referred to be an acid. If the substance’s pH is more than 7, it is regarded as a base. The substance is regarded as neutral when the pH is 7.
Properties of Acids and Bases
| S. No | Property | Acids | Bases |
| 1. | Taste | Sour in taste. | Bitter in taste. |
| 2. | Test with phenolphthalein | Acid turns phenolphthalein colourless. | Basic turns phenolphthalein pink. |
| 3. | Test with litmus paper | Blue litmus turns red. | Red litmus turns blue. |
| 4. | Whenever metals react | Acids and metals react to produce salt and H2 gas. (Only with metals in the activity series above hydrogen.) | Salts and H2 gas are also produced when bases interact with metals (apart from Al). |
| 5. | When carbonates are reacted | Such reactions produce carbon dioxide. | There is no reaction. |
Uses of acids
- Hydrochloric acid is used to remove rust from metals
- Acetic acid is diluted into vinegar, which is used in many household processes. Its main application for it is as a food preservative.
- Lemon juice and orange juice both include citric acid as primary ingredients. Additionally, it can be used to preserve food.
- Nitric acid is used in fertilisers, plastic, photographic films, explosives, and dyes.
Uses of bases
- The antidote for food poisoning, bleaching powder, and building construction all employ calcium hydroxide.
- Petroleum is refined using sodium hydroxide, and it is also used to make soap, textiles, and paper.
- Laxatives frequently contain magnesium hydroxide, popularly known as milk of magnesia. It is also used as an antacid, since it lowers any excess acidity in the human stomach.
Summary
Numerous items that are edible and non-toxic, such as grapes, oranges, turmeric powder, milk, and other items, are regarded as bases and acids. Taste and touch are two simple ways to recognise these natural acids and bases. However, some acids and bases are dangerous chemical reagents that cannot be distinguished by their physical qualities; for this reason, their chemical properties are crucial. These acids and bases mostly depend on the \({H^ + }\) ion and \(O{H^ – }\) ion concentrations. Acids are substances that cause protons (\({H^ + }\)) to be produced in water. Bases, on the other hand, are chemicals that generate more hydroxyl ions (\(O{H^ – }\)) in solution. The pH scale can be used to determine the numerical value of acids and bases.
Frequently Asked Questions
1. Are acids electrically conductive?
Ans. The flow of ions’ is what causes the conductivity. Acids split apart in solutions to produce (\({H^ + }\)) ions. Acids, therefore, carry electricity.
2. Why does dilute hydrochloric acid turn blue litmus red, but dry hydrogen chloride gas does not?
Ans. Dry hydrochloric acid does not produce ions, whereas diluted HCl does. Increasing the concentration of \({H^ + }\) in a solution causes it to change from blue litmus to red.
3. How does pH affect tooth decay?
Ans. The pH of the mouth decreases as we eat more foods that contain acid, which encourages the growth of harmful bacteria and leads to tooth decay. Therefore, tooth decay results from the mouth’s pH being decreased.