An Introduction to Matter
In addition to taking on various forms, the matter is composed of small particles. Because they are so small, it is impossible to see these particles with the human eye. We have mentioned below some of the various properties of matter. There are different states of matter can also be found. The three common states are solids, liquids, and gases. Atoms and other particles with mass and volume are included in the matter.
What do you understand by the Characteristics of Particles of Matter?
We are aware that every substance in our environment is composed of small matter particles. This means that these particles have some attributes and can affect the status of properties. These characteristics of the substance can be either physical or chemical.
For more help, you can Refer to our video in Science Concept. Check out the video Lesson for a better understanding.
What are the Characteristics of Particles of Matter
The particles of matter are very, very small.
- The particles of matter have space between them.
- The particles of matter are constantly moving.
- The particles of matter attract each other.
Let’s try to explain each characteristic of particles of matter with the help of an experiment.
The particles of matter are very, very small
You can demonstrate the extremely small size of matter particles by carrying out the following experiment with water and potassium permanganate.
- Put two or three crystals of potassium permanganate in a beaker with 100 ml of water, and mix. The potassium permanganate solution in water will be a dark purple tint.
- Approximately 10 ml of this solution should be taken out and placed in the second beaker with 90 ml of pure water. The second beaker’s potassium permanganate solution’s colour lightens slightly as a result of this dilution.
- Take 10 ml of this mixture and add it to the third beaker’s 90 ml of clear water. The solution’s colour will continue to lighten.
- Continue dilution of the solution in this manner 5–8 times.
- We obtain a potassium permanganate solution in water in this manner, but the water is still coloured.
- This experiment demonstrates how a small amount of potassium permanganate crystals may colour a significant amount of water.
- Therefore, conclude that each potassium permanganate crystal must contain millions of minuscule particles that continually divide into smaller and smaller particles.
The particles of matter have space between them
The experiment below, which uses water and sugar, can be used to demonstrate the gaps between the particles of matter.
- Have a 100 ml beaker ready.
- Mark the water level after adding half of the water to the beaker.
- Utilizing a glass rod, dissolve 50g of sugar.
- We’ll discover that the sugar solution’s level in the beaker is exactly where the water level was when the beaker was first filled.
- The crystals of sugar break down into incredibly small particles when they are dissolved in water. Since these sugar particles occupy the spaces between the different water particles, adding sugar to water does not change its volume.
- When sugar is dissolved in water, there is no change in volume, which indicates that there are voids between the water molecules.
The particles of matter are constantly moving
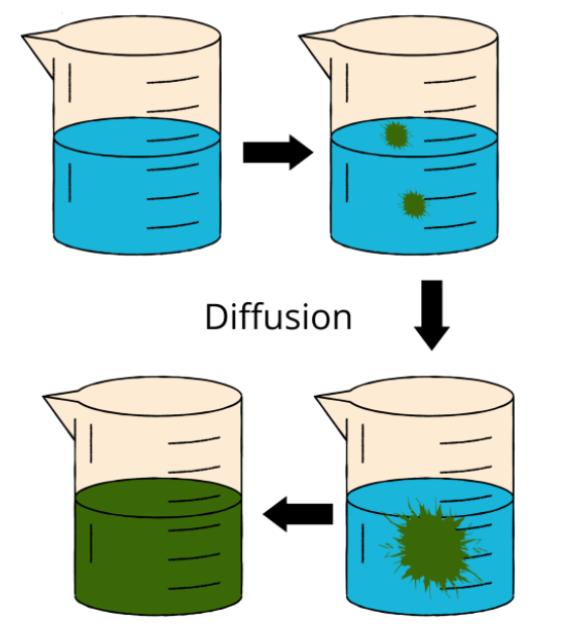
The investigations on diffusion and Brownian motion gave the particles their characteristic of continual motion.
- Water and red ink slowly combine, causing the water to eventually turn crimson.
- The movement of matter particles is demonstrated by this action.
The particles of matter attract each other
The forces of attraction that hold matter particles together are known as gravitational forces. Cohesion is the term denoting the force of attraction between particles of the same substance.
- When a piece of chalk, a cube of ice, and an iron nail are all hit with a hammer, the chalk is very easily broken into smaller pieces while the ice cube requires more energy to break, and the iron nail remains intact even when hit with a lot of force.
- This demonstrates that the force of attraction between the chalk particles is very weak, the force between the ice particles is a little stronger, and the force between the iron nail particles is quite strong.
Summary
There are three different types of physical nature in the world around us. Solid, liquid, and gas are these we breathe in air, which is a gas, and we drink water, which is a liquid. Because different types of matter contain varied amounts of inter-particle space, we have mentioned three possible states of matter. In this article, we studied the characteristics of solids, liquids, and gases. In a nutshell, this is how matter behaves physically in the universe.
Frequently Asked Questions
Question 1. What are the several forms that matter can take?
Solids, liquids, and gases are the three states in which matter can be found. Ice is a solid, water is a liquid, and steam is water in a gaseous state. Therefore, matter exists in all three states.
Question 2. How can you ascertain the material’s physical characteristics?
We are aware that everything we see is made of something. They take up space and have mass. It’s crucial to realise that not all matter has the same physical characteristics. One common illustration of this fact is the fact that while sand particles are insoluble in water, salt particles are. Therefore, these elements can be referred to as matter’s physical characteristics.
Question 3. What is Diffusion?
In matter, particles are constantly in motion. Diffusion is the term used to describe the natural mixing of particles from two different materials. The diffusion of these particles inside the substance speeds up as the temperature rises. It gets faster because as the temperature rises, the kinetic energy of the particles rises as well. They move quickly as a result.