Introduction
“An acid and a base react in chemistry to produce salt as a by-product. The negative ion (anion) of an acid and the positive ion (cation) of a base combine to form a salt. When an acid and a base come into contact, a neutralisation reaction takes place.” Table salt, composed of sodium chloride, is also referred to as salt. Most salts completely dissolve into negatively and positively charged ions when in solution or the molten state, making them excellent electrolytes.
What are the characteristics of salt?
- Salts are created when sodium and chloride combine.
- Ion bonds are electrostatic forces that bind ions together. They are drawn to one another by the opposite charges on the two ions.
- Saltwater is a good conductor of electricity, and the ionic compounds are neutral with no charge when they contain an equal number of opposite charges.
- Salts have an ionic character because they contain ions.
- Salt is a white, odourless, and salty tasting solid that is hard, crystalline, and brittle.
Explain the different types of salts
- Normal Salts-Electrical neutrality is present in typical salts. When acids and bases balance each other out, these salts are produced. Metallic ions completely replace hydrogen ions. Some examples, are NaCl, \(KN{O_3}\), \(CuS{O_4}\), etc.
- Basic Salt- A basic salt is the type of substance produced when a weak acid and a strong base react. This reaction creates a salt that is more basic. The pH of this salt is higher than 7. Sodium acetate (\(C{H_3}COONa\)), is a basic salt.
- Acidic Salt-Strong acids are neutralised by weak bases to form acidic salts. Such salt can dissolve in water and produce an acidic solution. Ammonium chloride (\(N{H_4}CI\)) is an acidic salt that is created when HCl, a strong acid, and \(N{H_4}OH\) (a weak base) react.
- Double Salt- A salt that contains two or more different cations or anions is referred to as a double salt. Examples of double salts include alums and Tutton’s salts.
- Mixed Salt- A salt that has a fixed ratio of two salts is called mixed salt. There is a common cation or anion in this mixed salt. Bleaching powder and sodium potassium carbonate are a couple of examples of mixed salts.
- Complex Salt- A complex salt is a substance made up of ligands surrounding a central metal atom in coordination bonds. Another name for this is a coordination compound. Because of the complex structure and the bonds between the cations and anions, this substance is known as a complex salt.
Explain the process of Neutralisation Reaction
When an acidic solution is treated with an alkaline solution or aqueous solution of a metal oxide, a salt is formed, and the solution becomes neutral. A neutralisation reaction occurs when \({H^ + }\) ions from an acid combine with \(O{H^ – }\) ions from the base of a metal oxide.
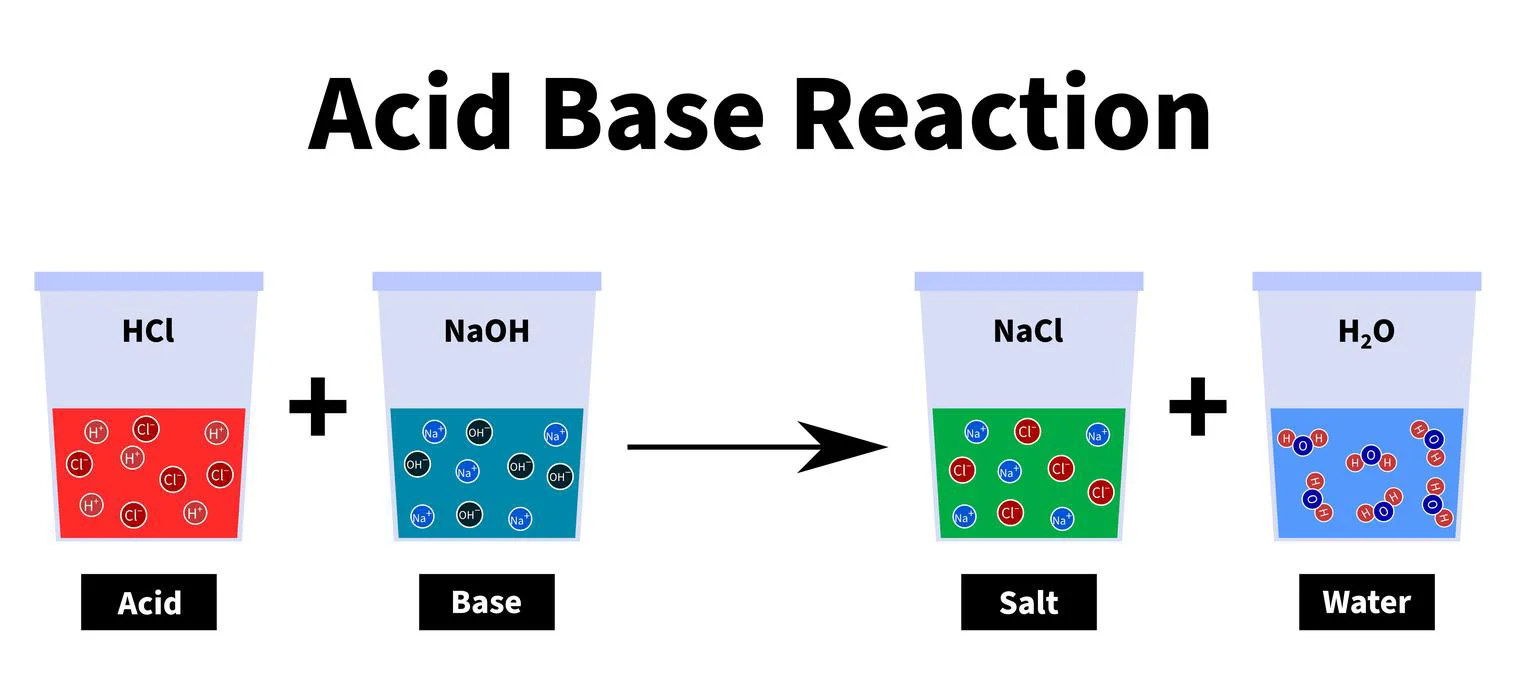
The chemical reactions shown below demonstrate the formation of salt.
\[HCl{\rm{ }} + {\rm{ }}NaOH{\rm{ }} \to {\rm{ }}NaCl{\rm{ }} + {\rm{ }}{H_2}O\]
\[{H_2}S{O_4} + {\rm{ }}Ca{\left( {OH} \right)_2} \to {\rm{ }}CaS{O_4} + {\rm{ }}2{H_2}O\]
Summary
Sodium chloride, also known as table salt, is a substance that we are all familiar with. We frequently season and preserve food with it. Other salt varieties and their applications, such as in the production of polyester fabrics, fertilisers, and dyes, are less well-known. Salts are frequently the result of an acid-base neutralisation reaction.
Frequently Asked Questions
1. How to tell whether something is neutral, acidic, or basic.
Ans. If the pH of a solution is lower than 7, it is said to be acidic. If the pH is 7, the solution is neutral; if it is higher than 7, the solution is basic.
2. Write five reasons why salt is important for the body.
Ans. Salt helps you stay hydrated, promotes good vascular health, balances electrolytes and prevents muscle cramping, supports a healthy nervous system, and improves sleep.
3. Is salt a chemical element?
Ans. Table salt is made up of the element sodium (Na) and chloride (Cl). Both elements are found bound together in nature as the compound sodium chloride, rather than occurring separately and freely.