Introduction
Atoms, which give all other matter in the universe its mass, volume, and resilience to survive changes in its physical state, are responsible for the matter’s mass and volume. Each type of matter, molecule, element, or even chemical, has a unique set of features that aid in understanding how that matter is used in everyday life. While the primary characteristics of matter are pressure, density, and volume, the primary characteristics of chemicals are toxicity, chemical stability, and the strength of their covalent bonds. As a result, there are many things to learn about the characteristics of each element and chemical complex.
What are Physical Properties?
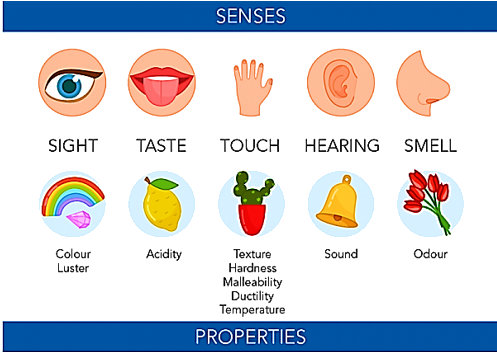
As is common knowledge, every element and form of matter has unique characteristics. Physical property is any attribute that can be measured and that also describes an object’s physical condition. A physical state can change through time, which is referred to as a physical state shift. Physical characteristics can also be seen. Meaning that any changes in the physical stuff are readily seen. Without affecting the substance’s identity, these qualities may be identified. Contrarily, this is not true of chemical attributes because the substance changes as a result of identification.
Example of Physical Properties
Recognition and measuring the properties of matter depend upon certain aspects, even though it does not need to undergo any changes in its identity. For instance, if it involves measuring the amount or substance then it is extensive physical property (by appearance)
- Volume
- Mass
- Length
- Shape
If it is not dependent on the amount of substance, then it is intensive physical property (by observing its physical state in extreme temperature)
- Melting point
- Colour
- Boiling point
- Density
Measurement of Physical Properties
For scientific study, measurements of physical attributes are required. Quantitative measures, as the name implies, are used to carry out the task and based on the physical properties (either extensive or intensive), a measurement is made. The SI units are used to express the measurements. The various physical quantities, together with their corresponding symbols and SI units, are displayed in the table below.
| Physical quantity | Symbols | Name of the SI unit | The Symbol for the SI unit |
| Length | l | metre | m |
| Mass | m | Kilogram | kg |
| Time | t | Second | s |
| Electric current | l | Ampere | A |
| Thermodynamic temperature | T | Kelvin | K |
| Amount of substance | n | Mole | mol |
| Luminous intensity | lv | Candela | cd |
Physical Properties of Elements
The physical properties of materials are determined by performing intensive material characterizations. We already know that two or more molecules may be combined to form an element. As a result, knowing its qualities based on the number of atoms it contains is simpler. We may learn about a substance’s density, electrical stability, and capacity to tolerate intense heat to determine its melting and boiling points. Understanding the characteristics of the elements is essential since it is beneficial in many ways. We can determine which elements share a particular attribute and which do not. Iron and copper, for instance, have similar characteristics but distinct ones. i.e., they can both conduct electricity. They cannot, however, be exposed to damp air.
Physical Properties of Materials
We have understood the properties of elements, but what about materials? Materials are nothing more than things like metals, ceramics, and polymers. Their differing densities and thermal characteristics set them apart from one another. Among a material’s characteristics are,
- Thermal conductivity
- Resistivity
- Density
- Melting point
- Corrosion resistance
Three Physical Properties of Water
Even water, which is measured in litres, has physical characteristics. Other than being placed in the container to acquire their form and volume retention, they experience no physical changes. Water has distinct physical characteristics:
- Temperature
- Colour
- Turbidity
- Taste
- Odour
Summary
Physical characteristics are observable, which means we can see them with our naked eyes. In contrast to chemical attributes, physical properties do not experience any changes to their physical state. There are two ways to observe physical qualities. Both extensive and intensive physical properties.
Frequently Asked Questions
1. What is a Physical Change?
Ans: Except for one or more physical features, a substance’s chemical properties remain unchanged. We refer to this as a bodily transformation. In other words, a substance is capable of taking on any shape, size, or structural modifications. Physical changes also include state changes, such as going from a solid to a liquid or from a liquid to a gas. Cutting, bending, melting, freezing, boiling, and dissolving are a few of the processes that result in physical changes.
2. What are the Chemical Properties of Matter?
Ans: Chemical characteristics are the measurements or observations of a chemical substance. Chemicals contain certain characteristics that can only be identified when the substance transforms into another sort of substance. For research objectives, chemical characteristics are very useful in differentiating molecules. Reactivity, flammability, and corrosion are a few of the characteristics. Reactivity is defined as the capacity to interact with other chemical compounds. Flames and chemicals react rapidly. Thus, the flame characteristic of many chemicals may be identified.
3. How do bonds Affect Physical Properties?
Ans: Chemical bonds are the electrical forces that hold ions and atoms together during the formation of molecules. These chemical bonds are responsible for the physical properties of matter like hardness, structure, melting, and boiling points. They also influence other properties such as crystal symmetry and cleavage etc. It is more difficult to break apart bonds that are stronger than they are. Hardness, higher melting and boiling points, and less chance of expansion are all caused by stronger chemical bonds.