Introduction
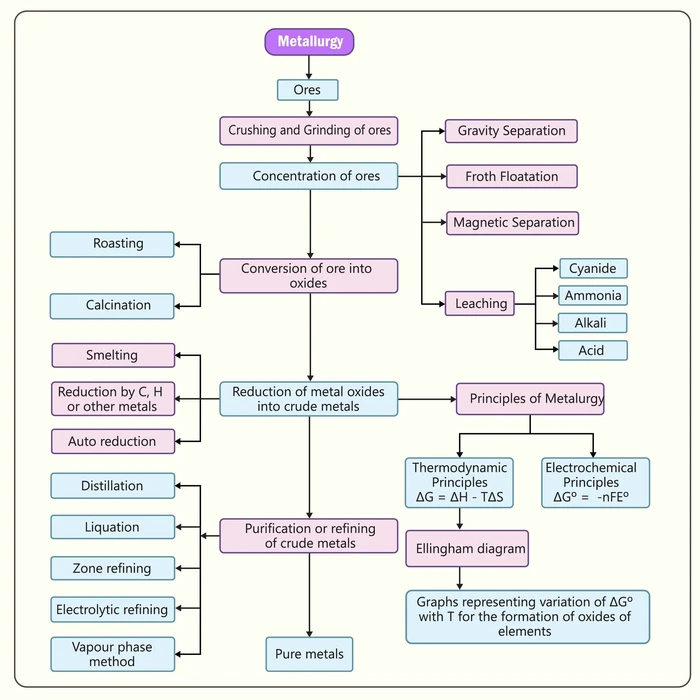
Calcination and roasting are two processes used to convert ores into oxides. Ores are a naturally occurring substance found in the earth’s crust. These ores are rich in minerals and valuable metals. Metals extracted from ore are required by applying lots of heat, either in the presence or absence of oxygen. Processes such as calcination and roasting are used to convert ores to oxides. The following is a step-by-step diagram for converting ores to oxide and extracting pure metals.
To produce oxide, raw ore is required. During this process of conversion, it expels volatile substances and gas. The removal of volatile impurities in solids and gases results in the extraction of metals from ores’ oxides. Furthermore, electrolytic refining purifies metal.
What Exactly is Calcination?
It is the process of converting carbonate ore to oxide below its melting point using heat in the absence of air or with a limited supply of oxygen. It is also referred to as the thermal decomposition process because it decomposes ore or solid substances without changing their chemical properties and only removes volatile and organic impurities. As an example:
\[ZnC{O_3} \to {\rm{ }}ZnO{\rm{ }} + {\rm{ }}C{O_2}\]
How would you define Calcination?
Calcination is a thermal or heat process that occurs when a solid substance, such as carbonate ore, is heated above its melting point in the absence or limited supply of oxygen. Calcination is derived from the Latin word Calcinare, which means ‘to burn lime’. As a result, the most commonly used ore limestone (calcium carbonates) produces quicklime in the absence of air or oxygen at temperatures ranging from 900 to 1050 °C (calcium oxide). The following is my reaction:
\[CaC{O_3} \to {\rm{ }}CaO{\rm{ }} + {\rm{ }}C{O_2}\]
Why Calcination is Necessary
Calcination is a method of purifying ores. Heating ores or solids to temperatures well below their melting points causes the decomposition or removal of volatile impurities, moisture and water, and organic matter. As an example:
1. Carbon dioxide removal from carbonated ores.
2. Hydrated molecules are extracted from bauxite and gypsum. The following is the reaction:
\[A{l_2}S{O_3}.2{H_2}O{\rm{ }} \to {\rm{ }}A{l_2}{O_3} + {\rm{ }}2{H_2}O\]
1. The extraction of volatile liquids from petroleum and coke.
2. In the preparation of zeolites, ammonium ions are removed.
What Exactly is Roasting?
It is a process of converting mainly sulphide ores into their respective metal oxides when subjected to heat in the presence of air or oxygen. It is one of the metallurgical processes.
How would you define Roasting?
The heating process of converting sulphide ores into metal oxides below their melting point in the presence of air or oxygen is known as roasting. The conversion of ore into oxides alters the chemical properties of the solid ores and results in the formation of a new product after impurities are removed. The roasting of zinc sulphide into zinc oxide results in the following reaction:
\[2ZnS{\rm{ }} + {\rm{ }}3{O_2} \to {\rm{ }}2ZnO{\rm{ }} + {\rm{ }}3S{O_2}\]
Why Roasting is Necessary?
Roasting is a process that converts sulphide ores into oxides by heating them to high temperatures in the presence of oxygen. It is used in the metallurgy process to extract metals or their oxides from ores by removing metallic, non-metallic, toxic, and moisture impurities in the form of volatile substances. The following impurities are removed during conversion:
1. Sulphur removal from sulphide ores.
2. Phosphorus and silicon in flux are removed. Flux is used to remove impurities in the form of slag.
What are the Main Differences between Calcination and Roasting
| Calcination | Roasting |
| In the absence or limited supply of oxygen or air, the ore is heated. | Heat is applied to ore in the presence of an excess of oxygen or air. |
| Carbonate ores are processed using this method. | This method is employed for sulphide ores. |
| Only decomposition occurs in this process, and oxygen is not involved in the reaction. | Oxygen is reacted with sulphide ores in this process. |
| Impurities such as organic matter and water are expelled. | Toxic impurities are removed. |
| Carbon dioxide is produced along with metal oxide. | Metal oxide and sulphur dioxide are both produced. |
| The process is also carried out in a reverberatory furnace. The furnace’s holes were kept closed. | It is accomplished in a reverberatory furnace. The holes in the furnace were kept open to allow oxygen or air to enter. |
Summary
Metals can be extracted using roasting and calcination processes. Metal-containing ores and minerals are not always present in the oxide form; in this case, roasting and calcination processes are used. Both processes convert the ore to its oxide form. This facilitates the extraction process. These only change when exposed to high temperatures. However, roasting occurs in the presence of oxygen, whereas calcination occurs in the absence of oxygen. During thermal decomposition, roasting produces new products, whereas calcination decomposes the solid substance. Both produce metal oxides, which can then be reduced to metals.
Frequently Asked Questions
1. Is there a physical process involved in calcination?
Ans. It is a decomposition process in which solid substances are broken down when high heat is applied. During this process, no new products are formed. As a result, it is only a physical change or process.
2. How do the calcination reactions take place?
Ans. Calcination reactions occur in retorts and furnaces. The ores or solid substances are stirred in this process to produce a uniform product.
3. Why are roasting and calcination done at temperatures below the melting point?
Ans. If ores are heated above their melting point, they will melt and mix with difficult-to-separate carbonate and sulphur impurities.