Introduction
There is no such thing as an ideal gas in the real world; hence, the term “ideal gas” is strictly a theoretical one. An ideal gas is made up of several independently spinning, point-like particles that do not collide with one another in any way. Because it strictly adheres to the ideal gas law, the concept of this ideal gas (theoretical gas) has practical or theoretical significance (or ideal gas equation). The molecules in a real gas do not behave according to the ideal gas law or ideal gas equation because they occupy the necessary space and interact with one another. Because of the interactions between its molecules, cool air at standard pressure and temperature acts like an ideal gas. However, as its temperature and pressure increase, its behaviour changes to that of a real gas.
What is an ideal gas?
An ideal gas, sometimes known as a perfect gas, is a type of gas that has physical properties that are in perfect alignment with an idealised link between pressure, volume, and temperature. Ideal gases are also often referred to as perfect gases. They perfectly follow the different gas laws such as Charles’ law. It follows the ideal gas law which is as follows:
PV = kT
where k is a constant.
Properties of an ideal gas
General characteristics of an ideal gas are as follows-
- Compressibility is one of the features of an ideal gas because its molecules have so much energy that they push each other closer together when compressed.
- Most collisions in an ideal gas with its container are elastic, meaning the gas retains all or almost all of its original velocity after impact.
- Because of their compressibility, perfect gases can assume the form of their storage vessel. The gas within the container has a total volume equal to the volume of the container itself.
- It is often considered that perfect gases do not interact with one another because of their inert behaviour.
- In an ideal gas, the individual molecules or particles are immobile, massless spheres.
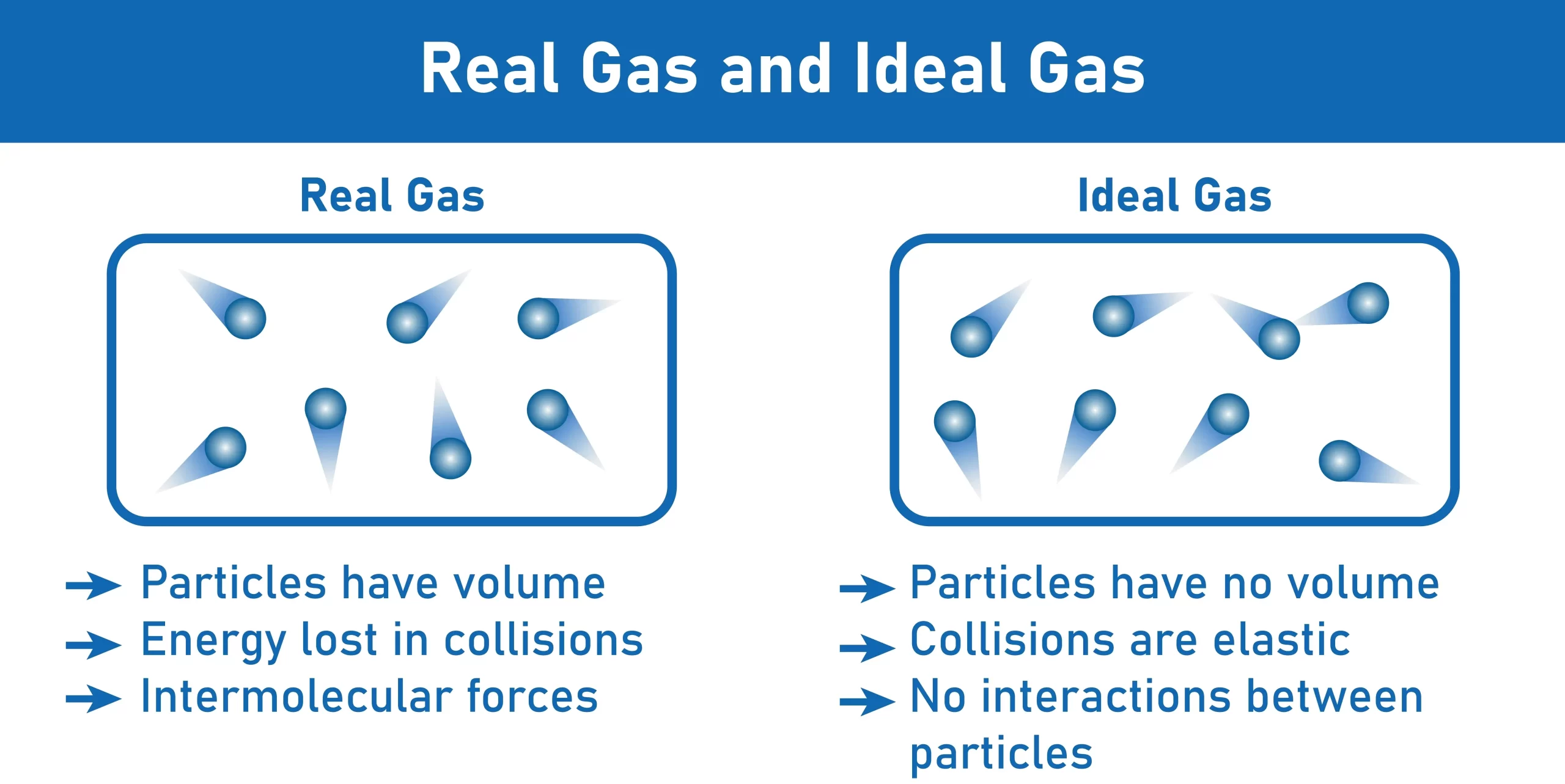
Real and ideal gases characteristics
What is real gas?
In contrast to their ideal and non-ideal counterparts, real gases do not have molecules that fill space and interact with one another. In reality, gas does not behave according to the ideal gas law or ideal gas equation. Real gases are therefore developed, modelled, or represented further by factoring in their molar weight and molar volume.
The gas law for real gases is called the Vanderwall equation. It is written as
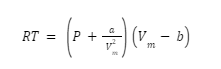
The variables a and b are determined empirically for individual gases.
Properties of real gas
General characteristics of a real gas are as follows-
- True gas has a measurable volume.
- In a genuine gas, the vast majority of collisions are non-elastic, whether with the container or other gas molecules.
- In a genuine gas, the molecules will interact with one another by either attractive or repulsive forces.
- When compared to an ideal gas, the pressure exerted by the molecules inside this system is lower.
- Molecules in a genuine gas can freely clash with one another.
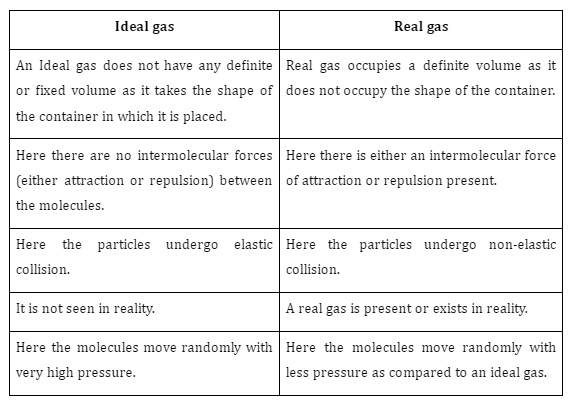
Difference between Ideal gas and Real gas
Summary
The equation PV = nRT, known as the ideal gas law, describes the theoretical behaviour of ideal gases. The molecules of an ideal gas travel at fast speed and in all directions simultaneously. Particles here interact with one another by elastic collision, as intermolecular forces are absent. Non-ideal gases, which include actual gases, are so named because they defy the ideal gas law and equation. Particles in a real gas can collide with each other in a way that is not elastic because the molecules are moving in random directions and the pressure is lower than it would be in an ideal gas.
Frequently Asked Questions
1. What is the kinetic theory of gas?
The kinetic theory of gases makes use of an extremely large number of submicroscopic particles, such as atoms and molecules, in order to characterise the molecular composition of a gas. According to the hypothesis, particles hitting one other and the container walls create gas pressure. The kinetic theory of gases describes temperature, volume, pressure, viscosity, thermal conductivity, and mass diffusivity. It describes all microscopic qualities.
2. What do the constants “a” and “b” depend on in the van der waals equation?
To calculate the attractive forces between gas molecules, scientists use the van der Waals equation, where the constant ‘a’ indicates the strength of the forces and the constant ‘b’ reflects the effective volume filled by the gas molecules. You may also hear it referred to as co-volume or excluded volume.
3. What is the compressibility factor?
The compressibility factor (Z) is a measure of the capability of a gas to compress under external pressure. It is determined by dividing the molar volume of a gas to that of an ideal gas at a constant temperature and pressure. The compressibility factor is equal to one in the case of a perfect gas.