Introduction
While the number of protons is merely the atomic number, the atomic mass of an atom is the sum of both its protons and neutrons. The letters A and Z can be used to denote these. Since it offers the key to the element’s existence, the atomic number is the concept that deals with such a periodic table element. It is only after interacting with this particular proton, which is primarily referred to as this hydrogen isotope’s protium, that the atomic and mass numbers are the same. Keep in mind, in particular, that while the atomic number remains constant, the mass number could change due to the presence of multiple isotopes. The elements are arranged in numerical order by atomic number.
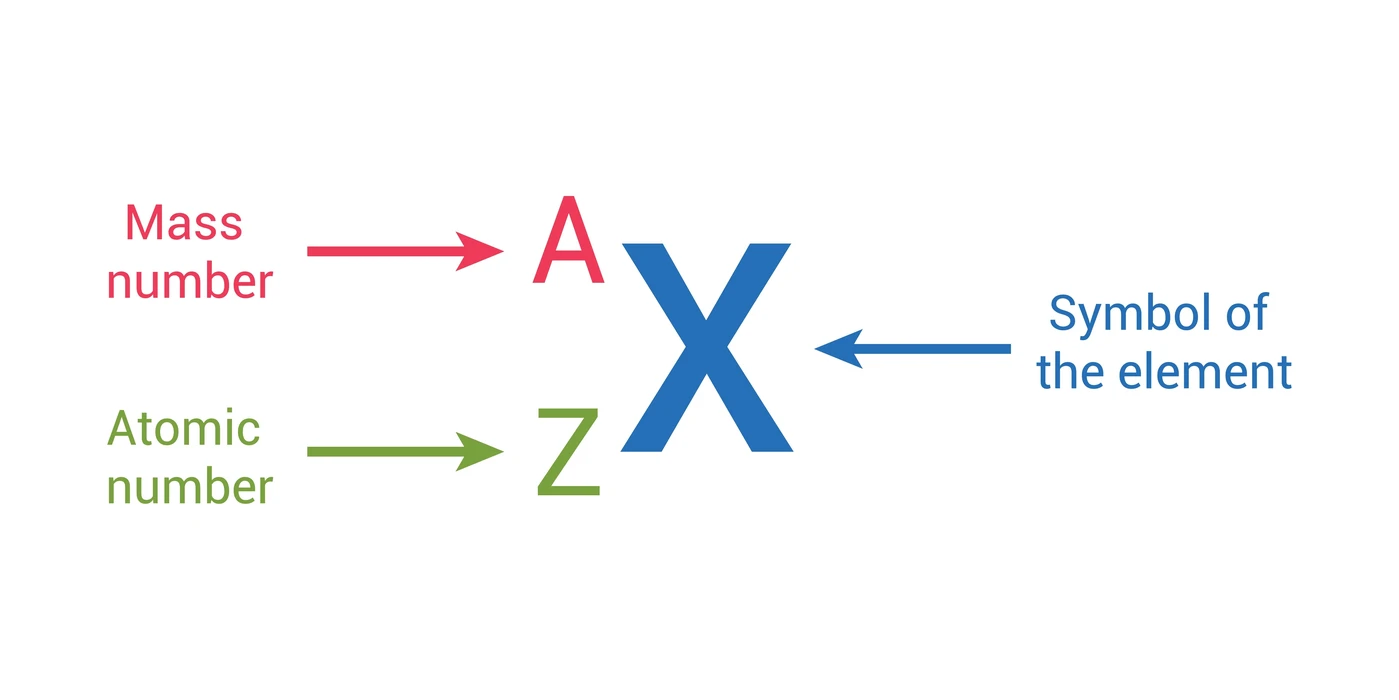
Define Atomic Number
The number of protons in the nucleus of an atom is the atomic number. This is denoted by the letter Z. The number of electrons that surround the nuclei is controlled by the number of protons. In a periodic table with ascending atomic numbers, compounds with similar chemical properties typically cluster in the same column. Different elements have distinctive atomic numbers. For example, all C atoms have an atomic number of sixes, whereas all O atoms have an atomic number of eights.
Define Mass Number
Rutherford proved that an atom’s nucleus, which is composed of protons and neutrons, contains perhaps the majority of the atom’s mass. The mass number refers to the total number of protons and neutrons in such an atom. Atomic mass units are used to measure this. To represent it, the letter “A” is frequently used. This has typically been accomplished by simultaneously adding both neutrons and protons.
For instance,\(Cl^{37}_{17} \) appears to have a mass number of 37. Its nucleus contains twenty neutrons and seventeen protons.
What is the difference between Valency, Atomic number and Mass number
| Valency | Atomic Number | Mass Number |
| The greatest amount of electrons that even an atom could lose, gain, as well as share, in addition to getting stable is referred to as valency. | An atomic no. is the no. of protons that exist in such an atom. | The mass no. within an atom is the total of its protons as well as neutrons. |
| The electronic arrangement of such an atom could be used to evaluate its valency. | The mass number has always been less than the atomic no. | The atomic no. is always greater than that of the mass number. |
| The no. of atoms does not affect valency. | No. of neutrons in an atom does not impact its atomic no. | The no. of neutrons inside an atom seems to not affect the mass no. |
| The no. of electrons does have a direct relationship with valency. | The atomic no. of isotopes seems to be the same. | The mass number of isotopes varies. |
| Elements are classified as monovalence, divalence, and trivalence based on their valency. | Isobars with similar atomic no. cannot exist. | The mass no. of isobars would be the same. |
Energy Levels of Atomic Orbital
When an electron reaches a certain energy level, it is more likely to be found in these regions than in other regions. Orbitals are the name for those sections. Orbitals with roughly similar energies have created sub-levels. The maximum capacity for each orbital is two electrons. The energy of such an electron in a specific atom may be determined solely by the primary quantum number. In order of increasing orbital energy are the following orbitals:
\(1s<2s=2p<3s=3p=3d<4s=4p=4d=4f\)
Summary
The mass number of an atom’s nucleus is an integer equal to the sum of the nucleus’ protons and neutrons. The atomic number, in contrast, is simply the number of protons. Even though their mass is so small compared to that of protons and neutrons, electrons are not counted when calculating mass because they have no impact on the value. The number of neutrons may change, even though the number of protons in such an element’s units remains constant. An electron appears to have very little mass. Therefore, an atom’s atomic mass is roughly equivalent to its mass no. The mass number represents the weight of an atom’s nucleus in atomic mass units.
Frequently Asked Questions (FAQs)
1. Is there a relationship between atomic mass and weight?
Ans: No, atomic mass is indeed the weight of an atom, while atomic weight denotes the weighted average of naturally produced elements.
2. Why does an atomic number refer to as a fingerprint?
Ans: The physical or chemical characteristics of an atom have been exclusively governed by the no. of electrons inside its nucleus, but often along with its nuclear charge: the nuclear charge would be an element’s specific “fingerprint,” as well as Z identifies the chemical components individually.
3. Why is it that a mass number is typically a whole number?
Ans: Since it is the total number of the particles, the mass no. is always a whole number. This varies from the atomic mass unit, which is well recognized, as well as written to 6 decimal points.

