Introduction
Chemical elements are the fundamental building blocks of chemistry, and everything around us is made up of elements. The periodic table is a tabular display of elements found in chemistry that are arranged by atomic number. A periodic table is an important tool for chemists, material scientists, and nanotechnologists because it provides so much information about the elements that it is easy to predict the physical and chemical properties of the elements. The periodic table demonstrates a fundamental but critical principle that the atomic number is responsible for chemical properties.
The periodic table contains how many elements?
The periodic table contains 118 elements organized in 7 rows and 18 columns. The rows (from left to right) are called ‘periods,’ and the columns (from top to bottom) are called ‘groups.’ All chemical elements have different physical and chemical properties, which change as you move in the periodic table. The arrangement is made so that the elements in the same column have similar properties. Surprisingly, only 94 of these 118 elements exist naturally.
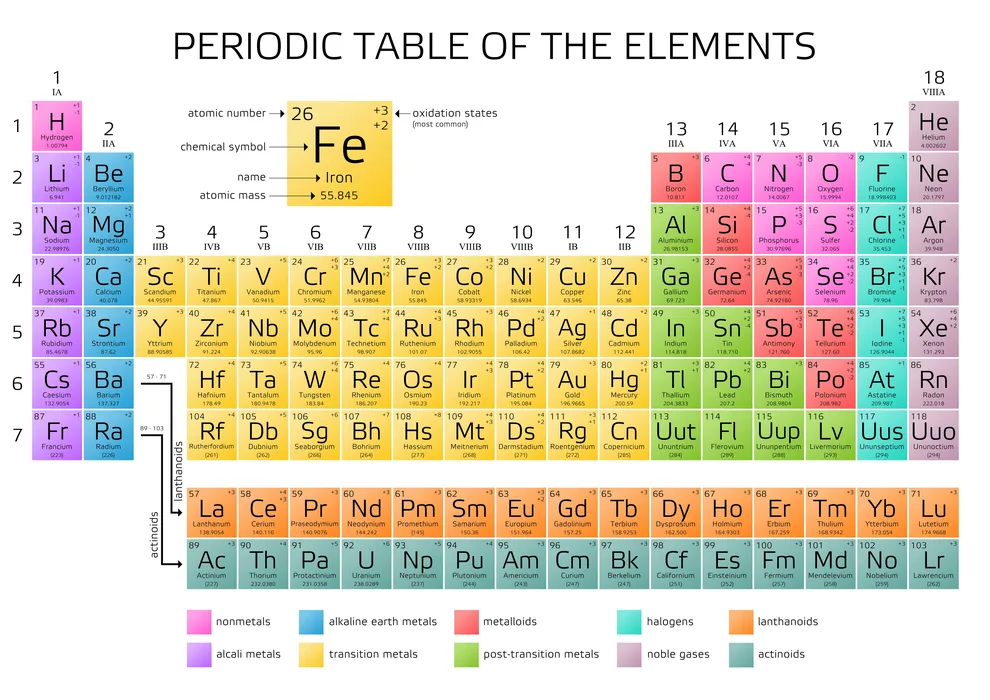
118 Elements Name and Symbols and Atomic Numbers in Chemistry
| Name of the Element | Symbol | Atomic Number |
| Hydrogen | H | 1 |
| Helium | He | 2 |
| Lithium | Li | 3 |
| Beryllium | Be | 4 |
| Boron | B | 5 |
| Carbon | C | 6 |
| Nitrogen | N | 7 |
| Oxygen | O | 8 |
| Fluorine | F | 9 |
| Neon | Ne | 10 |
| Sodium | Na | 11 |
| Magnesium | Mg | 12 |
| Aluminium | Al | 13 |
| Silicon | Si | 14 |
| Phosphorus | P | 15 |
| Sulphur | S | 16 |
| Chlorine | Cl | 17 |
| Argon | Ar | 18 |
| Potassium | K | 19 |
| Calcium | Ca | 20 |
| Scandium | Sc | 21 |
| Titanium | Ti | 22 |
| Vanadium | V | 23 |
| Chromium | Cr | 24 |
| Manganese | Mn | 25 |
| Iron | Fe | 26 |
| Cobalt | Co | 27 |
| Nickel | Ni | 28 |
| Copper | Cu | 29 |
| Zinc | Zn | 30 |
| Gallium | Ga | 31 |
| Germanium | Ge | 32 |
| Arsenic | As | 33 |
| Selenium | Se | 34 |
| Bromine | Br | 35 |
| Krypton | Kr | 36 |
| Rubidium | Rb | 37 |
| Strontium | Sr | 38 |
| Yttrium | Y | 39 |
| Zirconium | Zr | 40 |
| Niobium | Nb | 41 |
| Molybdenum | Mo | 42 |
| Technetium | Tc | 43 |
| Ruthenium | Ru | 44 |
| Rhodium | Rh | 45 |
| Palladium | Pd | 46 |
| Silver | Ag | 47 |
| Cadmium | Cd | 48 |
| Indium | In | 49 |
| Tin | Sn | 50 |
| Antimony | Sb | 51 |
| Tellurium | Te | 52 |
| Iodine | I | 53 |
| Xenon | Xe | 54 |
| Cesium | Cs | 55 |
| Barium | Ba | 56 |
| Lanthanum | La | 57 |
| Cerium | Ce | 58 |
| Praseodymium | Pr | 59 |
| Neodymium | Nd | 60 |
| Promethium | Pm | 61 |
| Samarium | Sm | 62 |
| Europium | Eu | 63 |
| Gadolinium | Gd | 64 |
| Terbium | Tb | 65 |
| Dysprosium | Dy | 66 |
| Holmium | Ho | 67 |
| Erbium | Er | 68 |
| Thulium | Tm | 69 |
| Ytterbium | Yb | 70 |
| Lutetium | Lu | 71 |
| Hafnium | Hf | 72 |
| Tantalum | Ta | 73 |
| Tungsten | W | 74 |
| Rhenium | Re | 75 |
| Osmium | Os | 76 |
| Iridium | Ir | 77 |
| Platinum | Pt | 78 |
| Gold | Au | 79 |
| Mercury | Hg | 80 |
| Thallium | Tl | 81 |
| Lead | Pb | 82 |
| Bismuth | Bi | 83 |
| Polonium | Po | 84 |
| Astatine | At | 85 |
| Radon | Rn | 86 |
| Francium | Fr | 87 |
| Radium | Ra | 88 |
| Actinium | Ac | 89 |
| Thorium | Th | 90 |
| Protactinium | Pa | 91 |
| Uranium | U | 92 |
| Neptunium | Np | 93 |
| Plutonium | Pu | 94 |
| Americium | Am | 95 |
| Curium | Cm | 96 |
| Berkelium | Bk | 97 |
| Californium | Cf | 98 |
| Einsteinium | Es | 99 |
| Fermium | Fm | 100 |
| Mendelevium | Md | 101 |
| Nobelium | No | 102 |
| Lawrencium | Lr | 103 |
| Rutherfordium | Rf | 104 |
| Dubnium | Db | 105 |
| Seaborgium | Sg | 106 |
| Bohrium | Bh | 107 |
| Hassium | Hs | 108 |
| Meitnerium | Mt | 109 |
| Darmstadtium | Ds | 110 |
| Roentgenium | Rg | 111 |
| Copernicium | Cn | 112 |
| Nihonium | Nh | 113 |
| Flerovium | Fl | 114 |
| Moscovium | Mc | 115 |
| Livermorium | Lv | 116 |
| Tennessine | Ts | 117 |
| Oganesson | Og | 118 |
The characteristics of the Periodic table
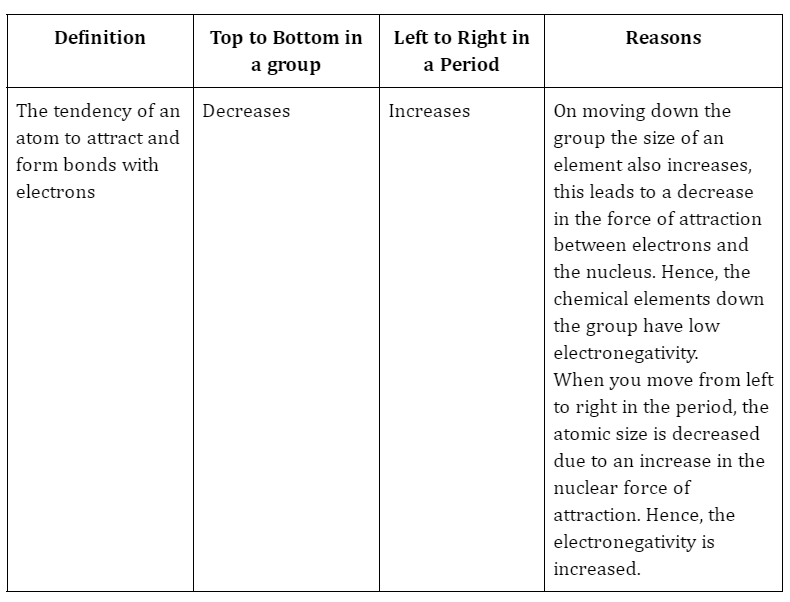
1. Electronegativity
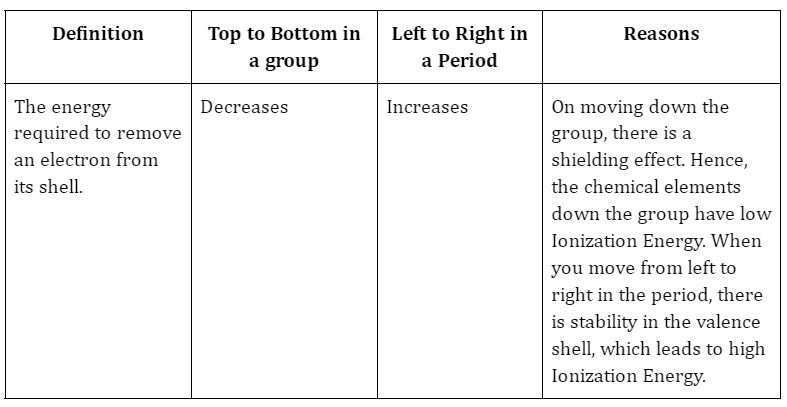
2. Ionization Energy
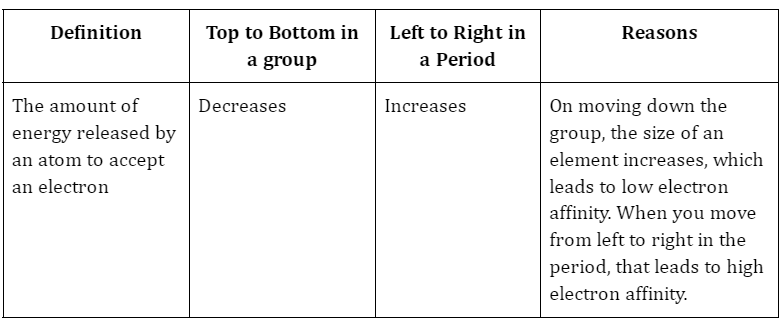
3. Electron Affinity
4. Atomic Radius
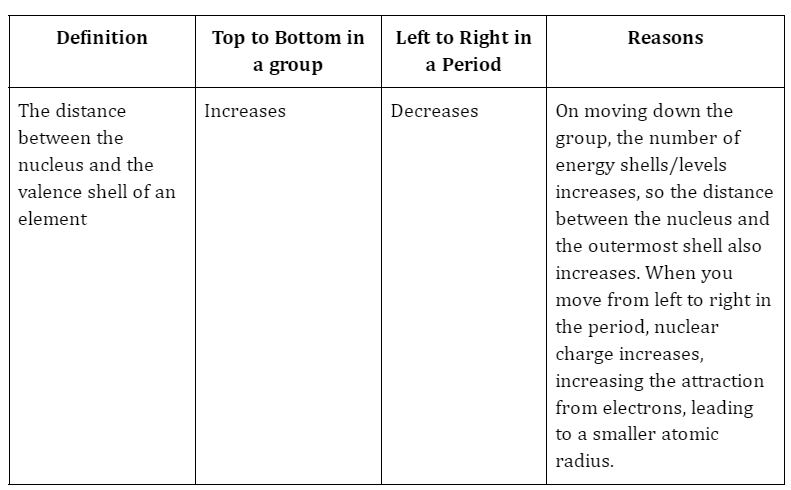
Summary
To date, mankind has discovered 118 elements. Only 94 of these occur naturally. These elements are represented in the periodic table, which has seven rows and eighteen columns. Columns represent groups, and rows represent periods. All elements are members of similar groups with similar chemical properties. The chemical properties of elements are determined by their atomic number. The number of protons in the atom determines the atomic number. This number also indicates the number of electrons in the atom. The chemical properties of an element are determined by the electrons in the valence cells.
Frequently Asked Questions
1. Why do elements in the same group share physical and chemical properties?
Ans. The physical and chemical properties of elements depend on the number of valence electrons. Elements present in the same group have the same number of valence electrons. Therefore, elements present in the same group have similar physical and chemical properties.
2. Why are noble gases also called inert gases?
Ans. Noble gases are also known as inert gases because their electron configuration is the most stable. Because the valence shells are completely filled, they cannot lose or gain electrons.
3. Why ionization energy is always positive?
Ans. Electrons in an atom are bounded by forces of attraction from the nucleus. And we know the electron will be attracted to the nucleus due to the charge difference. This means the energy that is provided to take out an electron from its shell. This is why the ionization energy is always positive.

