An Introduction to Matter
- The matter is anything that takes up space and has mass.
- All matter, whether it be living or non-living, is claimed to be composed of the five fundamental components (panch tattva) of air, earth, fire, sky, and water.
- The two ways that modern scientists categorize matter are based on its physical and chemical characteristics.
- Material is divided into three categories: solids, liquids, and gases based on its physical characteristics.
- The matter is divided into elements, compounds, and combinations based on its chemical makeup.
Physical nature of Matter
Tiny particles make up matter. The three fundamental categories of matter—solids, liquids, and gases—are based on how these particles are arranged. They are sometimes referred to as the physical states of matter. Additionally, this classification is based on variations in several physical characteristics, including mass, volume, form, stiffness, density, and particle arrangement.
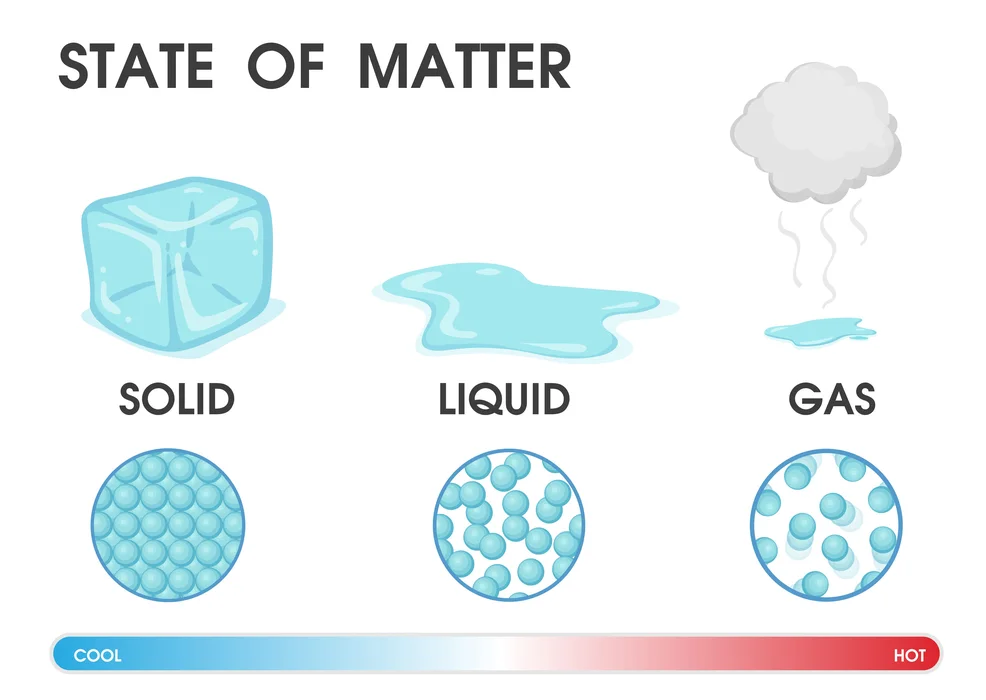
Physical Nature of Matter: The Solid-State
In general, all solids have a fixed volume with little compressibility, a fixed shape, and defined borders. When a force from outside the solid is applied, the solid usually keeps its shape. This demonstrates how stiff they are. Solids, however, may break under force.
Characteristics of Solids
- Solids have a definite shape
- Solids have fixed volume
- Solids cannot be compressed
- Solids have high density
- Solids have negligible kinetic energy of the particle
- Solids do not show the property of diffusion
- Solids cannot flow
Physical Nature of Matter: The Liquid State
As we’ve seen, fluidity or particle motion is hardly noticeable, while stiffness is at its highest in the solid state. Both of these properties differ when the substance is liquid. In terms of the physical nature of matter, liquids are less rigid than solids and also exhibit considerably greater molecular motion. The presence of weaker inter-particle forces accounts for both of these properties in the liquid state.
Characteristics of Liquids
- Liquids do not have a fixed shape
- Liquids have a fixed volume
- Liquids cannot be compressed much
- Liquids show fluidity but not rigidity
- Liquids are less dense
- Particles can diffuse easily in a liquid state
Physical Nature of Matter: The Gaseous State
The gaseous state has the most inter-particle gaps out of the three states described by the Physical Nature of Matter. The different particles are kept together as tightly as possible in the gaseous state by inter-particle interactions. As a result, stiffness is at its lowest and fluidity is at its highest.
Characteristics of Gases
- Gases do not have fixed shapes
- Gases exhibit maximum fluidity
- Gases are highly compressible
- In the gaseous state, the kinetic energy of the particles is very high
- Gases diffuse rapidly
Summary
There are three different types of physical nature in the world around us. Solid, liquid, and gas are these we breathe in air, which is a gas, and we drink water, which is a liquid. Because different types of matter contain varied amounts of inter-particle space, we have mentioned three possible states of matter. In this article, we studied the characteristics of solids, liquids, and gases. In a nutshell, this is how matter behaves physically in the universe.
Frequently Asked Questions
1. What are the differences between solids, liquids and gases?
| Solids | Liquids | Gases |
| Have strong intermolecular force. | Weak intermolecular force. | Very weak intermolecular force. |
| Have definite shape and volume. | Do not have a definite shape, but have a definite volume. | Neither have definite shape nor definite volume. |
| Have high density. | Have low density. | Have very low density. |
| Solids can not be compressed. | Liquids can be compressed. | Gases are highly compressible. |
2. What do you understand by matter?
Ans. The matter is anything with mass that takes up space. Atoms are the minuscule constituent parts of matter. Matter exists in three different states. Gas, liquid, and solid.
3. What are physical property and chemical properties?
Ans. A substance’s physical property is a quality that can be seen or quantified without affecting the substance’s identity. Colour, density, hardness, and melting and boiling points are examples of physical qualities. The capacity of a substance to go through a particular chemical transition is described by its chemical property.

