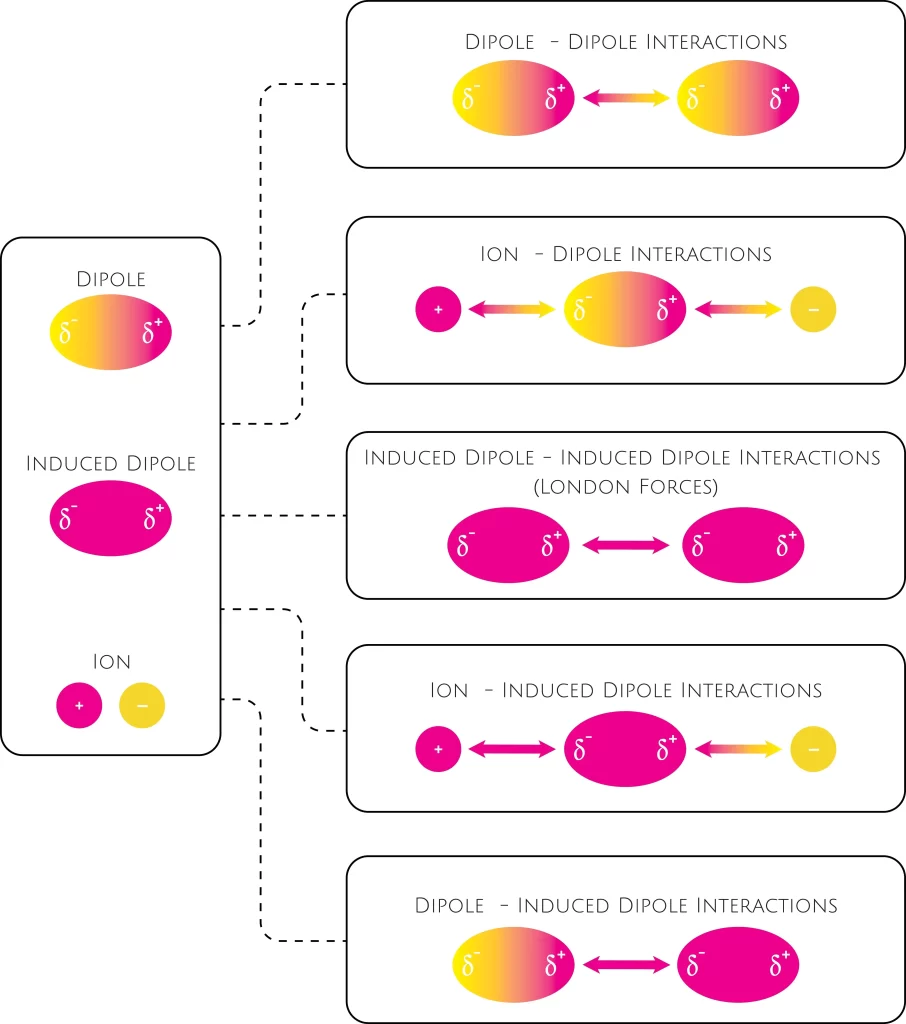
Introduction
The terms nucleophile and electrophile were coined by Christopher Kelk Ingold in 1933 to replace A. J. Lapworth’s anionic and cationic terminology. The term “electrophile” is a result of merging the words “electro,” denoting electrons, and “philes,” indicating a sentimental attachment. The word nucleophile comes from the combination of the Greek word ‘Philos,’ which means buddy, and the word nucleus. The field of chemistry relies heavily on these two concepts. Many organic reactions rely on the presence of these chemical substances. Electrophiles and nucleophiles, whose opposing behaviour is the impetus for many chemical processes, are well-known entities. Thus, it is clear that these definitions are crucial for a full comprehension of chemical processes.
Overview of Nucleophiles
Chemical species known as nucleophiles are able to give up a pair of electrons. They give up electrons because they are a species with an abundance of electrons. The word nucleophiles can be broken down into its component parts to denote any species that shows a preference for the nucleus. They are referred to be Lewis bases because of their ability to donate the pair of electrons they already possess. Lone-pair-of-electron species and negatively charged species are examples of neutral species. These chemical species are the ones that give up their pair of electrons during a chemical reaction, resulting in the creation of covalent bonds.
Nucleophilicity, which is comparable to the term basicity, describes the degree to which specific nucleophiles can transfer the pair of electrons. The element ammonia is a good example of a nucleophile because it has an unpaired electron.
Examples of Nucleophiles
As nucleophiles are negatively charged or they are species that contain lone pair of electrons. Some examples of nucleophiles can be given as,
- All the halogen anions,\(B{r^ – },C{l^ – },{I^ – }\)
- Cyanide,\(C{N^ – }\)
- Ammonia, \({NH_3}\)
- Hydroxide ion
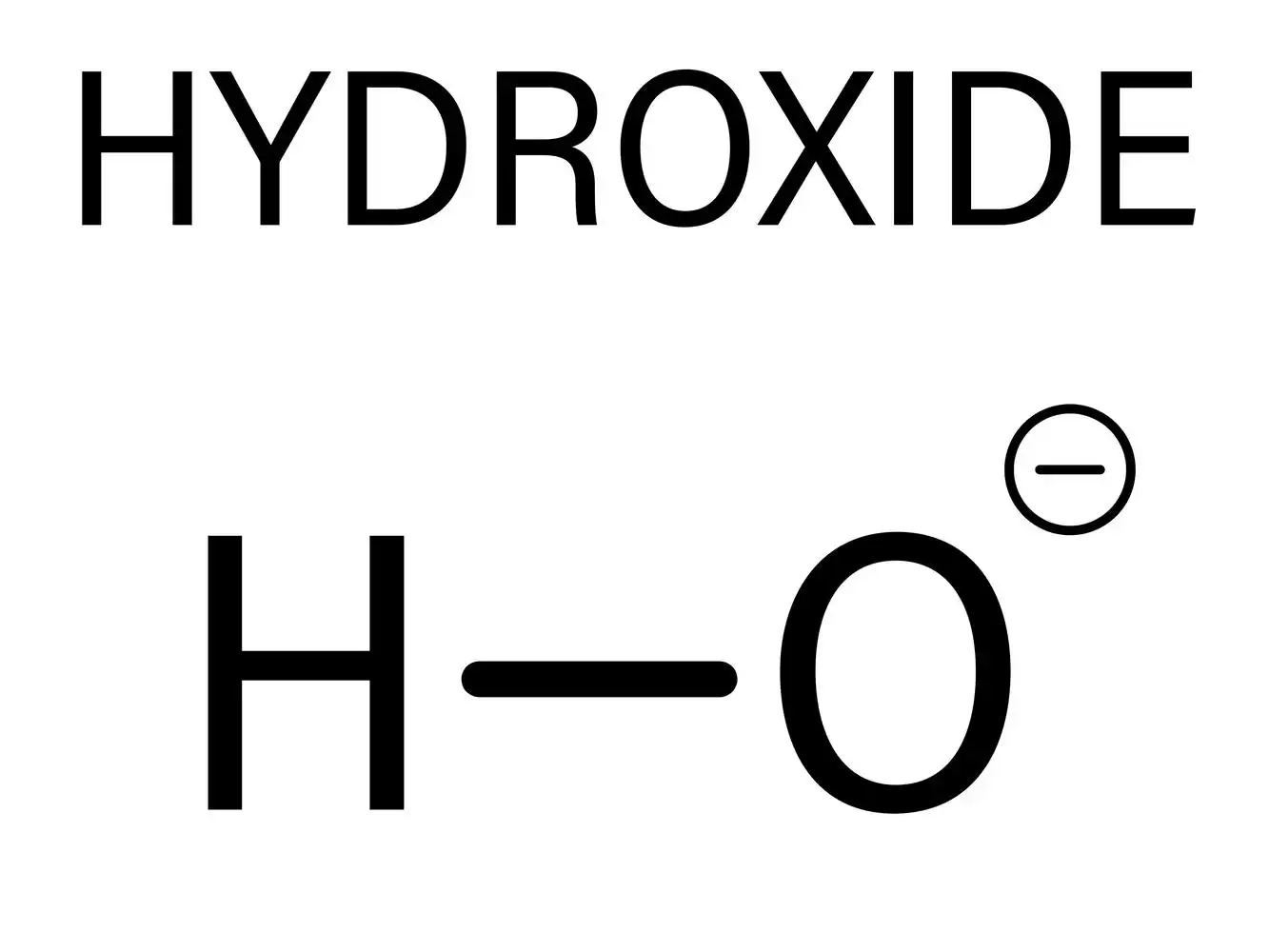
Structure of hydroxide ion
Features of Nucleophiles
This section elaborates on some of the key characteristics that nucleophiles must process.
- There must be a net negative charge on a nucleophile, or it must have a lone pair of electrons if it is an electrically neutral species. Therefore, nucleophiles are typically anions.
- A decreased electronegativity is required of nucleophiles in order for them to donate electron pairs effectively, so that they can be considered an inventive nucleophile. As a result, nucleophiles are often composed of less electronegative species.
- The strength of nucleophiles can be affected by the solvent used in a chemical reaction, especially if the solvent is polar or protic and acts upon the nucleophiles.
- Polar solvents can create hydrogen bonds with nucleophiles’ lone pairs of electrons, decreasing the likelihood that the nucleophiles will donate their electrons to other molecules.
- The rate of nucleophilic reactions can be slowed if nucleophiles are sterically hindered.
Overview of Electrophiles
Chemical substances having electron deficiency are called electrophiles. As a result, it attracts electrons towards itself since it has high electronic efficiency.
Two individual words, “electro” and “philes,” make up the phrase “electrophiles.” Electrophiles is a compound term that means “electron-loving species.” These substances might be either positively or neutrally charged chemical species. These compounds will take part in addition and substitution processes involving electrophiles. When they like interacting with a partner electron, electrophiles are referred to be Lewis acids. For this reason, the creation of a covalent bond is contingent upon the presence of these chemical species, which are able to accept a pair of electrons as part of a chemical reaction.
Examples of Electrophiles

Features of Electrophiles
We’ll go through what makes a good electrophile, and what makes a bad one, in more depth below.
- In order to accept electrons from reacting nucleophiles, an electrophile must be positively charged or have an unoccupied orbital.
- To attract electrons, an electrophile needs to have a weak link, hence electrophiles typically have weak polar bonds.
- Because of steric hindrance, electrons cannot be transferred to electrophiles if they are too close to other electrophiles. Thus, an electrophile should not be sterically hindered.
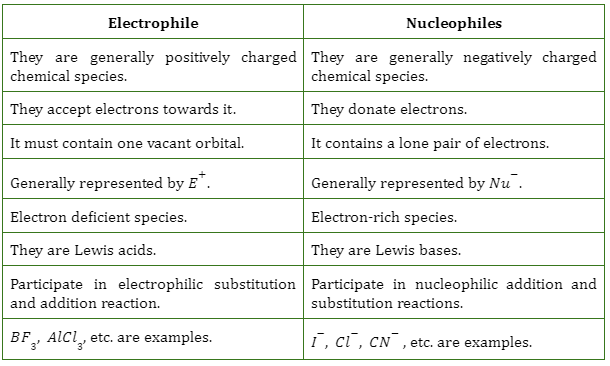
Difference Between Electrophiles and Nucleophiles
Some of the differences between electrophiles and nucleophiles are tabulated in the following table.
Summary
Chemical reactions take place by the donation and acceptance of electrons from one species to another species. Electrophiles and nucleophiles are two important chemical species that are necessary to undergo a chemical reaction. Electrophiles are the species that are positively charged or it a container back in the orbital to acceptive electrons. While nucleophiles are the chemical species that negatively charge lone pair of electrons so that they can donate this pair of electrons to another species. Some of the examples of electrophiles are \({BF_3}\) ,\({AlCl_3}\)
etc. And examples of nucleophiles are, \(C{N^ – },O{H^ – }\), etc. The important features of electrophiles and nucleophiles are affected by factors such as charge, electronegativity, steric hindrance, etc.
Frequently Asked Questions
1. Which of the following is the most powerful nucleophile in a nonpolar solution: I, Br, Cl, or F?
Ans. Since the strength of a nucleophile increases with increasing electronegativity in nonpolar solutions, fluorine (F) is the most potent nucleophile in such a medium. As far as electronegativity goes, fluorine is the winner. That’s why it’s the strongest nucleophile there is.
2. What is the effect of solvent on nucleophilicity of a molecule?
Ans. Nucleophilic replacements benefit from more polar solvents since the nucleophile is generally an ionic molecule and needs to be dissolved in a polar solvent. Ions may be more stable in polar solvents than in others.
3. Why is \({BH_3}\) an electrophile?
Ans. \({BH_3}\) is an electrophile since the boron atom has an empty p orbital and an electron deficiency. Thus it easily acts as an electrophile.