Introduction
The widely recognized nebular theory postulated that a massive cloud of dust including hydrogen and other gases created the solar system, including the planet earth. Small earthly particles like iron and nickel were created as a result of the earth’s contraction, rotation, and lowering of temperatures. The planet was created approximately 4.5 billion years ago after millions of years of precipitation and accumulation. Since then, the earth’s temperature has decreased, causing the crust to become more fragile. However, the earth’s interior core is still hot and igneous.
A short note on Prehistoric Earth’s Origin of Life
There were numerous theories about the origins of life on Earth.
- According to the Panspermia theory, some scientists thought life originated from spores that came from outer space, while others hypothesized that it originated from decomposing materials like dirt, straw, etc (Spontaneous generation theory). Different experiments were carried out by various scientists, and they all disproved the hypothesis of spontaneous generation.
- Later in 1953, Oparin and Haldane advanced the theory that life emerged from pre-existing non-living organic molecules like RNA, DNA, and other similar molecules because of abiotic chemical reactions.
- Numerous studies have suggested that RNA came before DNA, even though it is still unclear what replicating molecule was the earliest. Because RNA molecules can self-replicate and are simpler than DNA, they are considered autocatalytic.
Precambrian Life
The Precambrian period includes the Archean and the Proterozoic eons from 4.6 billion years to 542 million years. Most of the life that existed during the Precambrian period were prokaryotic organisms. Microfossils that looked like stromatolites and cyanobacteria from the Precambrian epoch first revealed the presence of life about 3.8 billion years ago (layered mounds). Additionally, the absence of oxygen in the early atmosphere rendered primitive organisms anaerobic. However, when cyanobacteria developed photosynthesis, it added oxygen to the atmosphere.
Eukaryotes, which have a nucleus, cytoskeleton, organelles, and mitotic spindle, first evolved around two billion years ago. It was once thought that endosymbionts like mitochondria and chloroplasts descended from bacteria. The evolution of eukaryotes benefited greatly from these endosymbiotic relationships.
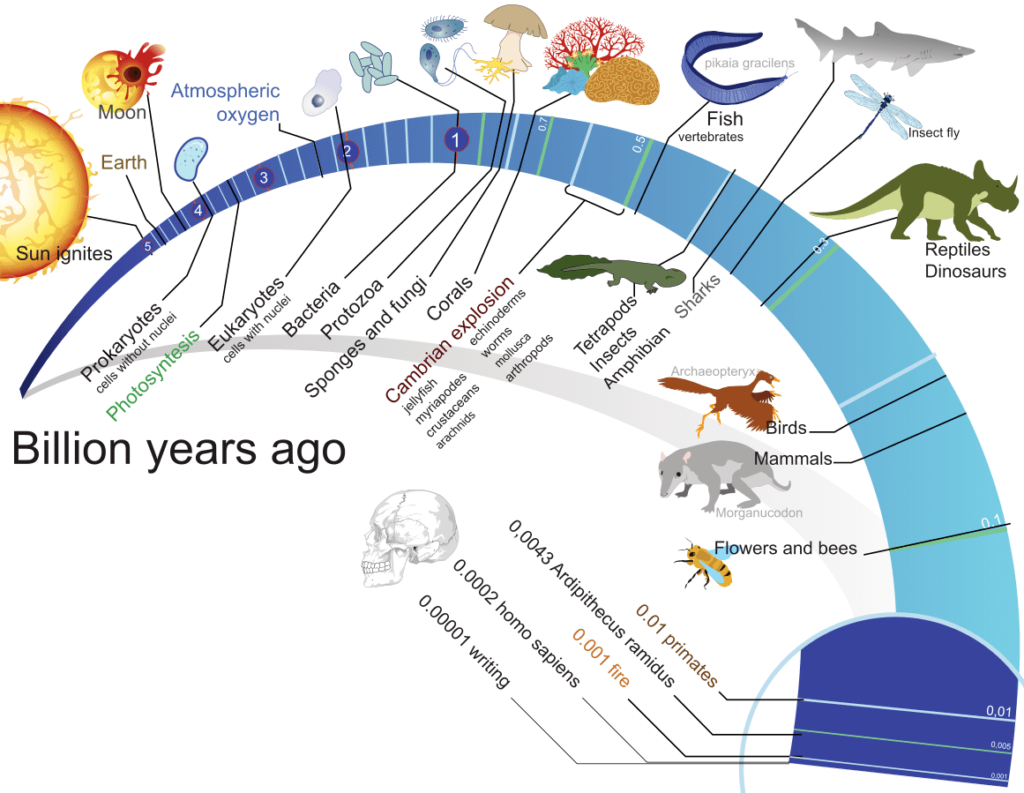
Cambrian Explosion- Origin of Animal Diversity
The surge in the diversity of multicellular organisms during the early Cambrian epoch, which began 540 million years ago, is known as the Cambrian explosion. Tens of millions of years before the early Cambrian epoch, the first multicellular living forms began to appear as fossils. These ancient animals have diverse body designs from those of living creatures today. They vanished and were replaced by modern-day animal body types in the Cambrian fauna.
Evolution of Land Plants
The evolution of land plants from a green algal ancestor is a significant event in the history of life since it caused profound changes in the earth’s environment and the formation of the entire terrestrial ecosystem. The formation of land plants and the divergence of the four main surviving clades (liverworts, hornworts, mosses, and vascular plants) may have taken place during the late Ordovician and Silurian periods, according to evidence from fossil spores found in the mid-Ordovician.
Formation
The majority of researchers concluded that the earliest life form and subsequent other life forms on earth appeared as a result of chemical evolution, or the production of molecules. The Nebular theory, developed by Immanuel Kant and Pierre Laplace, postulates that planets are formed by a cloud of hydrogen and helium. Clouds were created by tiny particle collisions, and the planet itself was created through accretion.
Evolutionary Milestones
- Life’s history is represented by several milestones. For instance, unicellular organisms first appeared on Earth, and ever since then, diversification has led to the emergence of complex living forms. These living forms will eventually go extinct and be replaced by other creatures.
- Complex living forms evolve as a result of this evolution. According to some fossil research, numerous species began to independently become multicellular around 1 billion years ago, and animals started to grow hard portions in their bodies to survive on the earth.
- Dinosaurs were the most prevalent class of creatures on the planet for millions of years. For a considerable amount of time, they dominated the prehistoric landscape before a disaster led to the extinction of dinosaurs.
- The Great Apes, from which humans emerged, was the next significant event. Human evolution is still clearly visible, although it has not yet reached its conclusion.
Presence of Humans
- The most well-known species, Homo sapiens, is a descendant of hominids, the first creatures that resembled humans.
- According to several fossil records, archaeological findings, and embryological research hominids are thought to have diverged from other primate species in the southern and eastern African areas 2.5–4 million years ago.
- As a result, they have bipedalism in common (the ability to walk on two legs).
- Additionally, as hominids evolved and adapted to their habitats, their brain sizes grew. Around 2.3 million years ago, Homo habilis, the earliest human-like hominid, had a brain size of 650–800 cc and started using stone tools.
- Fossils discovered in Java in 1891 revealed the existence of Homo erectus, the next stage of human evolution, some 1.5 million years ago. They have a 900cc larger brain due to evolution. Then they began to migrate from Africa to Eurasia, where they started to learn how to make fire and develop defenses.
Summary
The widely recognized Nebular Theory postulated that a massive cloud of dust, including hydrogen and other gases, created the solar system, including the planet Earth. The majority of scholars concluded that the earliest life form and subsequent other life forms on earth appeared as a result of chemical evolution, or the production of molecules. Dinosaurs were the most prevalent class of creatures on the planet for millions of years. The most well-known species, Homo sapiens, is a descendant of hominids, the first creatures that resembled humans.
Frequently Asked Questions
1.What is Coal’s Formation Process? List the Types of Coal.
Ans. The layers of dead plants and animals underwent physical and chemical changes as a result of pressure and heat. Deposits rich in carbon were created as a result of this. Different forms of coal include lignite, bituminous coal, and anthracite.
2.Explain Index Fossils?
Ans. Index fossils are fossils that are used to identify geologic formations with broad regional distributions and short time scales. These fossils are numerous, dispersed, limited in geological time, and unique.
3.What Factors led to the Earth’s Changes?
Ans. Physical changes such as mountain development, tectonic movements, volcanic eruptions, climate changes, and biological changes on the planet resulted from the evolution of new life forms.
4.What are the Necessary conditions for Life to Sustain on Earth?
Ans. The necessary conditions for life to sustain on earth are as follows,
- Proper distance from the sun
- Presence of water and the atmosphere
- Existence of the lithosphere and biosphere
- Ideal temperature ranges (around 17 degrees Celsius).