Introduction
A mixture is formed by physical means constituting two or more types of components. A mixture is usually of two types: homogeneous and heterogeneous mixture. Heterogenous mixtures are non-uniform mixtures which do not have a single phase throughout. The word “homogeneous” has roots in Latin and Greek. The words homo and gene mean “same” and “kind,” respectively. Hence, if a mixture is described as homogenous, all of the mixture’s components are the same.
What are Homogeneous Mixtures?
Substances in a homogeneous mixture are uniformly distributed across the entire medium. To put it another way, a sample taken from any point within a homogeneous mixture will have the same composition and yield the same results.
If, for instance, a solid-liquid solution is divided in half along its volume, each half will have the same amount of liquid medium and dissolved solute.
Some essential characteristics of a homogenous mixture are-
- A pure substance is made primarily of one type of thing, such as sodium metal or hydrogen gas. Homogeneous mixtures and solutions are often referred to as pure materials since their constituent parts are difficult to identify.
- The constituents of a homogenous mixture can be split into their original entities through physical means such as evaporation, distillation, etc.
Classification of homogeneous mixtures:
Homogenous mixtures are usually categorized based on their phase, as follows:
Liquid Homogenous mixture
A solution is a homogenous liquid mixture of two or more substances. When a solute needs to be dissolved, a solvent is used. One such example is water. A solute is primarily a component that is present in lesser quantity. An everyday example is sugar. For illustration, consider the mix of water, sugar, and flavour.
Solid Homogenous mixture:
A classic example of a solid homogenous mixture is an alloy. It is solid at room temperature and has the same composition throughout.
Gaseous Homogenous mixture
A gaseous homogenous mixture has two or more two gases dispersed evenly. For example, the air is a gaseous homogenous mixture with various gases, such as oxygen, carbon dioxide, nitrogen, etc, distributed evenly in the entire space.
Applications
Homogeneous mixtures have various uses and can be found in many commonplace objects, ranging from man-made polymers to naturally occurring solids like stone.
- Homogeneous mixtures find widespread use in the food business. Products like salad dressings, sauces, and soups are all examples of homogeneous combinations. All of these concoctions result from blending various substances in calculated proportions to get a specific taste or feel.
- The majority of drugs, including cough syrups and eye drops, are homogeneous mixtures. These concoctions are made by combining various active components in a calculated proportion.
- The manufacturing sector also makes use of homogenous mixes. Paints, adhesives, and lubricants are a few examples of products that use homogeneous mixes. To achieve the necessary uniformity or performance, these mixtures are made by combining several elements in a calculated proportion.
- Chemical reactions and chromatography are just two examples of experiments that need uniform solutions. To provide the intended effect, various compounds are combined in a predetermined proportion to produce these mixes.
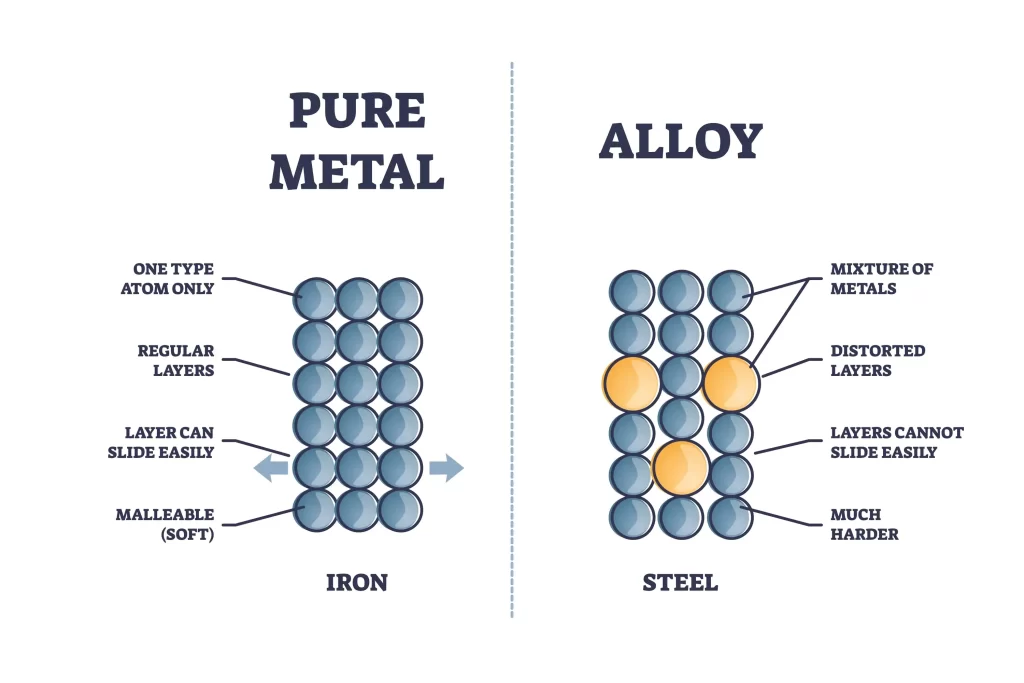
- Alloys of precious metals like gold, silver, and platinum are produced using homogeneous mixes in the jewellery industry. Many types of jewellery, from rings to necklaces, are crafted from these alloys. The uniform combination of metals results in an appearance and feel that can’t be replicated with just one metal.
- Homogeneous mixes are utilised to produce lightweight and robust alloys for the aerospace sector. Materials from these alloys are used to fabricate parts for aeroplanes, such as landing gear and fuselage pieces. The alloy produced by the uniform combination of metals is robust and lightweight, making it a good candidate for aeroplane use.
Summary
A homogeneous mixture is a uniform mixture having a clear composition and recognizable characteristics. The components of a homogeneous mixture are invisible. An illustration of a homogeneous mixture is a salt solution dissolved in water. It is impossible to distinguish the salt from the water when it dissolves since it spreads completely throughout the water, producing the same component of the solution.
Frequently Asked Questions
1. What are the indications that a mixture is homogeneous?
Ans. Consider the sample size of a combination to determine its nature. It is heterogeneous if there are multiple phases of matter or distinct locations in the sample. The mixture is homogeneous if its composition seems uniform no matter where you examine it.
2. How can a homogenous mixture be separated?
Ans. Homogenous mixtures cannot be separated by physical means, such as filtration or distillation.
3. Do solutions usually include homogeneous mixtures?
Ans. Although all homogeneous mixtures are solutions, not all solutions are homogeneous mixtures. A homogeneous mixture is a solution if there is only one phase present. When a solute is fully dissolved in a solvent, no undissolved particles remain as a result.

