Introduction
The matter may be found on Earth in three different states: solid, liquid, or gaseous. Every matter is composed of incredibly tiny building components called atoms and molecules. These particles in a solid are strongly attracted to each other and vibrate in place without passing by each other. Despite not being as strong as it is in a solid, there is still an attraction between the particles in a liquid. The proximity and frequent movement of the particles in a liquid allow them to slip past one another. There is only a weak attraction between gaseous particles. They are continually moving and spread out compared to the particles in a solid or liquid. Here the particles do not interact when they collide; they just strike and bounce off of one another. This article provides a thorough understanding of the concept of matter of space between particles and attraction between the matter particles with specific instances.
Particles have Space between Them
Let’s observe a small activity to determine whether the particles are separated from one another; this is explained below.
Experiment
Take a beaker filled with 100 ml of water and then add 20 gm of sodium chloride (table salt) into it. Make sure to swirl the water with a glass stirring rod until all the salt has completely dissolved. The salt will dissolve, and then we will get a solution.
Observations
It has been observed that even after 20 gm of salt has been dissolved in 100 ml of water, the level of the water doesn’t actually rise. This displays how free and interparticle space-containing water atoms are. This area is known as the interparticle or intermolecular space. In this interparticle area, the salt granules that were scattered have settled.
Particles of Matter are Constantly Moving at Random
Diffusion and Brownian motion demonstrate that particles are moving.
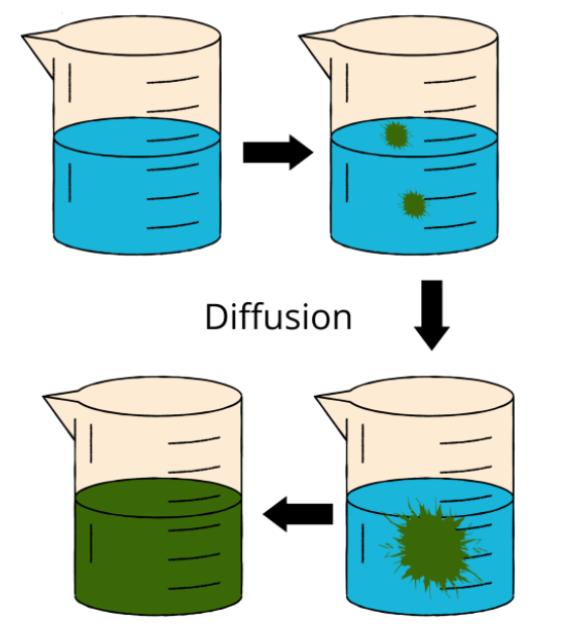
Diffusion
A material will mix and disperse with another substance through a process called diffusion while its particles are in motion. To homogenize the mixture, this process is repeated. For instance, the ink diffuses in the water as a result of the random movement of water and ink particles. Ink migrates from areas of higher concentration to regions of lower concentration at a pace that is inversely associated with the liquid diffusion rate of the ink. Diffusion occurs in liquids, solids, and gases, however, it occurs more rapidly in gases and less efficiently in solids.
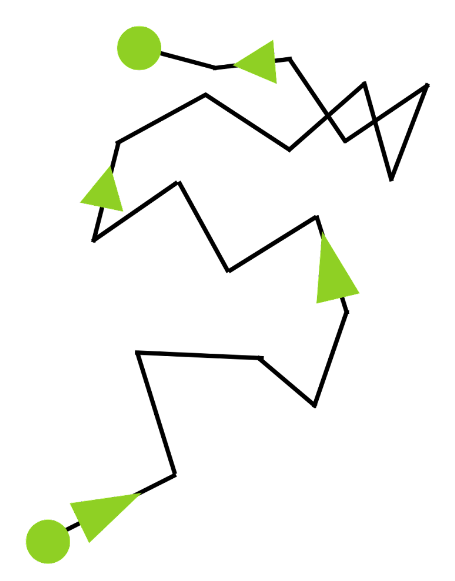
Brownian Motion
Brownian suspended several pollen grains in water and then examined them under a microscope. He saw that the pollen grains were moving in a zigzag pattern. The movement is significantly more evident when the water is warmed. Water is made up of randomly migrating atoms. As a result, the moving atoms frequently strike the pollen grains, causing them to migrate. As an example of Brownian motion, the pollen grains are travelling in this manner.
Particles of Matter Attract Each Other
A force acting on the particles of matter holds them together. Some substances crumble into powder, while others form tiny crystals, and still, others are challenging to separate. The strength of the force of attraction varies from one type of substance to another, depending on that substance. This is done so that the force of attraction between the particles can keep the particles inside them. This interparticle force of attraction exists in all substances that cause the attraction of particles. Therefore, to break objects, we must defeat the force of attraction. A varying amount of strength is necessary depending on the chemical.
Examples show that breaking a chalk is easier than breaking a nail. This illustrates how
various material particles have varied levels of attraction. The attraction between particles of the same material is referred to as “cohesion.”
Summary
The particles are separated by a certain amount of space, in which the gaseous form of matter has the largest inter-particle space among the three states of matter. Interestingly, the interparticle gaps that exist in various types of matter are what give rise to the three states of matter, and therefore the density of different states of matter increases from a gas to a solid state (for example water vapor to water and then ice).
Frequently Asked Questions
1. Define Matter.
Ans: A component that is made up of several types of particles, takes up space, and has motion is referred to as “matter.”
2. What Constitutes Matter?
Ans: Matter is made up of atoms, which are made up of electrons, protons, and neutrons.
3. How to Develop the Model of Particles of Gas and Liquid?
Ans: By compressing a flexible plastic container with a balloon on top, we can imitate the gas particles. We can also try to squeeze a water-filled container as part of their modelling of liquid particles.