Introduction
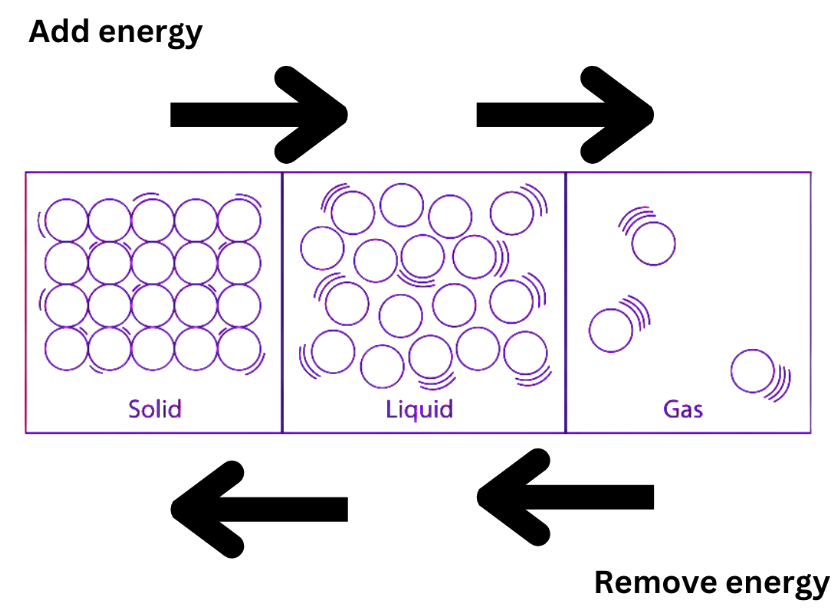
We use a variety of materials for our necessities. Some are naturally occurring, while others are the result of human labour. Natural resources are plentiful as a result of the abundance of numerous resources in nature. Carbonization contributes significantly to global coal production. Coke is the primary by-product of high-temperature carbonization; approximately 4% of the total input coal is converted into tar and crude benzol (light oil), with significant amounts of gas also produced. The following useful products are produced by processing coal without the use of air:
1. Coal and Gas
2. Coke
3. Tar from coal
Coal and Gas
Coal gas is a combustible vapour fuel extracted from coal that is piped to customers. Town gas is a broader term for gaseous fuels produced for commercial sale and community use. In some places, it is also known as manufactured gas, syngas, or producer gas.
Depending on the techniques used to create it, coal gas is a mixture of gases such as, and, as well as volatile hydrocarbons, with minor quantities of non-caloric gases such as and as impurities.
In the nineteenth century, coal gas, which was primarily a by-product of the cooking process, was widely used for lighting, cooking, and heating. The rise in natural gas production coincided with industrialization and urbanisation, and the by-products, coal tars and ammonia, served as key chemical feedstock for the chemical industry.
Coal, oil, and gas production
Coal gas is produced when coal is heated in an enclosed chamber with no air.
When bituminous coal is heated to 400 °C, it relaxes and coalesces, releasing water steam, rich gas, and tar. Crude oil, coal, and gas are examples of fossil fuels. Over centuries, coal oil was produced from the remains of dead trees and other plant debris. Crude oil and natural gas were extracted from dead sea creatures.
Composition of Coal gas
Coal gas is a gaseous mixture of \({H_2}\), CO, and \({H_4}\) that is produced and used as fuel by destructive distillation (burning bituminous coal in an inert atmosphere). Steam is occasionally introduced to combine with the heated coke, increasing gas production. It is primarily composed of \({H_2}\) and \({H_4}\), with trace amounts of other hydrocarbons, carbon monoxide (a lethal gas), carbon dioxide, and nitrogen. It functions as both a fuel and an illuminant.
Uses of Coal
Coal is used for a variety of things, including
1. Electricity generation: Coal is primarily used to generate electricity. When thermal coal is burned, steam is produced, which powers turbines and generators that generate energy.
2. Liquification, as well as Gasification, is the process by which coal is burned and crushed with steam to produce town gas for home heating and lighting. It is liquefied to produce synthetic fuels that are similar to petrol and diesel.
3. Chemical, as well as other industries: Syngas can be used to create chemicals such as methanol and urea. Coal is widely used in the paper, textile, and glass industries. Coal is also used to make carbon fibre and other speciality components such as silicon metals.
Define Coke and State its Properties
Coal is distilled destructively to produce a high-carbon product. Coke is known as a virtually pure form of carbon due to its high carbon concentration. It’s a greyish-black solid that’s hard and porous. It is used as a reducing agent and a fuel in mineral extraction and steel production.
1. It has nearly pure carbon properties.
2. It is hard, porous, and black.
3. When it burns, it produces no smoke.
Uses of Coke
1. In metal extraction, it is used as a reducing agent.
2. Used in the steelmaking process.
3. It can also be used to generate energy.
Define Coal Tar and State its Properties
Coal Tar is a by-product of the coke manufacturing process. It has the same colour as coke, but it is a thick, viscous liquid with a foul odour. It is used to make synthetic colours, pharmaceuticals, fragrances, plastics, paints, and other products. Not only that, but it is also capable of producing naphthalene balls.
Coal tar was discovered in 1665 and used for medicinal purposes in the 1800s. Itchy skin, UV sensitivity, allergic reactions, and skin discolouration are all potential side effects. It’s unclear whether taking it during pregnancy is safe for the baby, and it’s not usually recommended to take it while breastfeeding.
Coal Tar Applications
Coal tar is primarily used to produce coal-tar products, as well as refined chemicals such as coal-tar pitch and creosote. Coal tar treatments have long been used to treat skin conditions such as eczema and dandruff.
Summary
Coal, as a solid carbon-rich material that is black/brown and occurs in stacked sedimentary layers, is one of the most important fossil fuels. In the future, coal liquefaction techniques could provide readily available, non-polluting fuels and chemical raw materials. Analytical criteria play a significant role in the four coal refining processes described above. Fumes, oils, and tars, as well as soluble but low-volatile extracts, pitches, and cokes, must all be investigated.
Frequently Asked Questions
1. Why do fossil fuels pollute the atmosphere?
Ans. The combustion of fossil fuels produces significant amounts of air pollution and pollutants such as \({H_2}\), CO, and \(S{O_2}\), all of which can contribute to climate change by increasing the greenhouse effect.
2. What exactly is petroleum?
Ans. Petrol, plastic, and other chemical compounds are derived from petroleum, a mineral oil found beneath the ground or in the sea. Petroleum by-products include wax, kerosene, LPG, petrol, lubricating oil, and diesel.
3. What are the different types of coal?
Ans. The least valuable and softest type of coal, with the lowest carbon concentration, is peat. Because of its high moisture content, it is unsuitable for use as fuel.
Anthracite coal is the best available. This type of coal is also known as hard coal. It contains the most carbon. It only produces a small amount of smoke.
Lignite is slightly firmer than peat, but it is still quite soft. It contains more carbon than peat.