Introduction
A lattice crystal has a Frenkel Defect if an ion or maybe an atom occupies a location that shouldn’t be there. Yakov Frenkel, a Russian scientist, is honoured with this name. One atom in the crystal intentionally moved its position, creating the void. Due to the presence of valencies and self-interstitial defects, this flaw is frequently referred to as a dislocation defect. Crystal lattice sites become vacant when little cations are displaced from their usual positions.
Formation of Frenkel Defect
Frenkel defect can arise in crystal due to following reasons:
- A cation exits the lattice as well as becomes interstitial.
- A void is produced in the lattice.
- The uprooted cation settles in a nearby position among the other cations as well as anions.
Occurrence of the Imperfections in Solid Crystal
One of the most distinguishing features of crystals over amorphous substances is their ionic alignment. Yet, as no material exists in a perfectly ordered state, lattice flaws are always present.
Defects can be thought of as imperfections. The science of solid-state chemistry investigates the imperfections of solid crystals. A perfect crystal unit might contain anything from one atom to an infinite number of atoms. Crystal faults are the names given to certain imperfections in a crystal. Hence, imperfections in the crystal structure are known as crystallographic defects. Crystallographic flaws come in a wide variety of shapes and sizes, from points to lines to planes. Frenkel faults are localised imperfections. Several different types of flaws may be found, including:
- Impurity Defects
- Stoichiometric Defect,
- Frenkel Defect
- Schottky Defect
Frenkel Defect Example
Some examples of Frenkel Defect are as follows:
- Silver Bromide
- Silver Chloride
- Potassium chloride
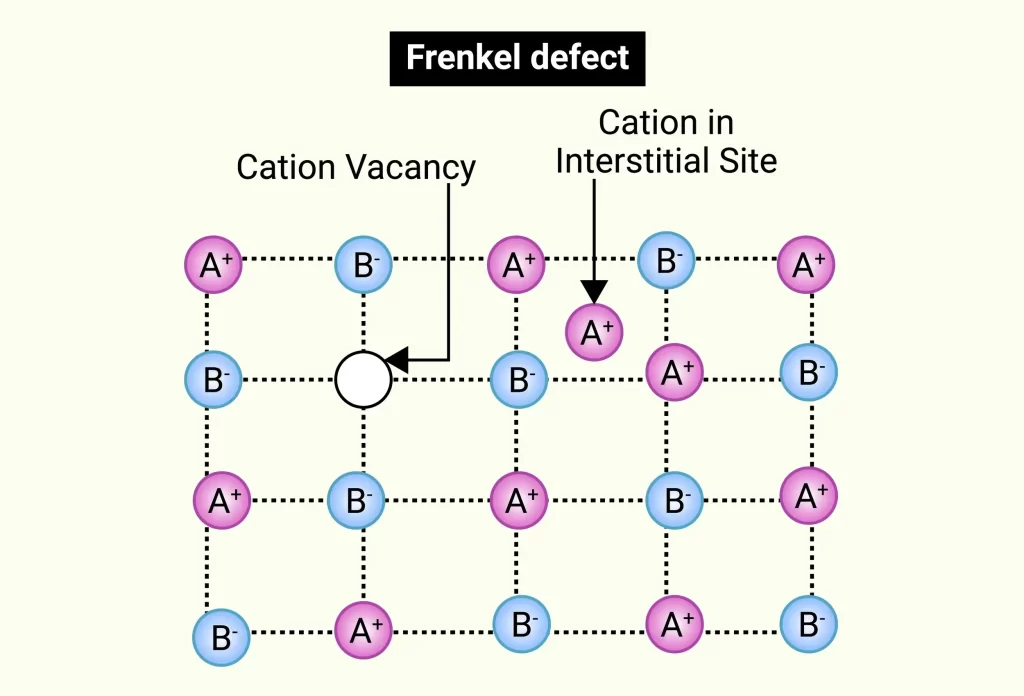
Frenkel Defect Diagram
Reasons for the Frenkel Defect
Due to the unit cell features at repeated fixed distances, defects are common in solid-state formations because the placements of molecules or atoms in crystals are predetermined. Particle irradiation is a major source of these flaws. The structure of a crystal is often imperfect and unstable. To put it another way, the equilibrium does not exceed the detection limit since the enthalpy of production is higher than at any other period during particle irradiation. Materials containing displaced cations are also susceptible to the formation of these flaws spontaneously.
Calculation of Number of Frenkel Defects
The Frenkel defect may be calculated using the following formula:
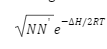
Where,
N = normally occupied positions
N‘ = no. of available positions
H = Frenkel Defect’s enthalpy formation per atom
R = gas constant
T = temperature
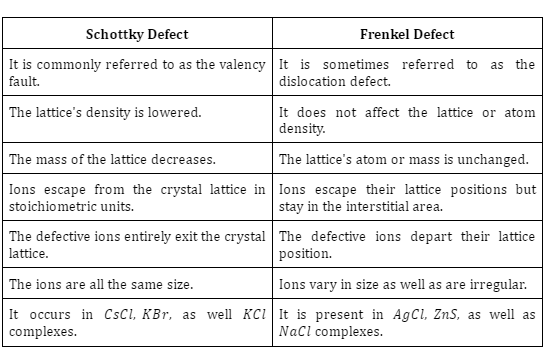
Difference between Schottky and Frenkel Defect
Some key differences between Schottky and Frenkel Defect are:
Summary
A Frenkel defect occurs when an ion or atom occupies a vacant site in a crystal lattice. A site vacancy is created when a cation moves, and it fills in at a neighbouring site. Particle irradiation results in this flaw. The Frenkel flaw has no effect on the chemical properties. It does nothing to increase the crystal’s density or make it non-conductive. Frenkel defect is proportional to the number of occupied and unoccupied areas, as well as the temperature. Because of the movement of ions, Frenkel defect is sometimes referred to as a dislocation defect.
Frequently Asked Questions
1. Why can the s group elements not show Frenkel defect?
The atomic defect indicated necessitates a low coordination number and molecule-friendly crystal lattices. The defect does not exist in alkali metal halides because cations and anions are almost the same size and cations cannot be accommodated in interstitial regions.
2. What is special property of AgBr crystal?
Frenkel and Schottky defects are both seen in AgBr because to its intermediate radius ratio. AgBr displays Schottky defects when both anions and cations are absent from the crystal lattice. The Ag+ ions in a material are very mobile and tend to move across the lattice. This causes them to exhibit the Frenkel flaw as well.
3. Does Frenkel Defect affect physical properties of a crystal?
The defect has an immediate effect on ion migration, but it has no effect on the solid-state structure’s volume or density. Thus, it does not affect the physical properties of crystal. As atoms pack closely together, stresses develop between them, leading to lattice growth. This growth more than compensates for the contraction of the lattice caused by vacancy.