Introduction
The rate of reactions is a word used to describe the rate at which a reaction occurs. The concentration of the reactants is a reliable indicator of the reaction rate. One can think of the rate of a reaction as being directly proportional to the production of the product and inversely proportional to the decreasing concentration of the reactant. The pace of reactions can be calculated with relative ease by tracking the shift in concentration.
For example, the combustion reaction on cellulose occurs rapidly, typically in a matter of seconds, and the rusting of iron is a relatively sluggish process that requires a long time to develop.
What is the Order of Reaction?
The Order of Reaction describes the correlation between process rate and species concentration in a chemical reaction. First, the rate expression (or rate equation) of the reaction must be determined in order to determine its order.
So, the order of a reaction is the exponents to which the reactant species are raised and it depends on the rate of that particular reaction. The order is achieved from the rate law. For example, in a reaction,
aA+bB⟶P
The rate equation is given as:
\(rate = k{[A]^x}{[B]^y}\)
The order of the reactant A is x fand for the reactant B, the order from the equation is y, Thus the overall order of the reaction,, n=x+y .
Characteristics
- The sequencing of a reaction has certain defining features. There is no guarantee that the response order will always be a whole number. It is clear from the equations themselves that the amount of a reactant used to make a product is crucial. However, this feature is not always present. As a result, the amount of reactant present in a reaction is irrelevant to the outcome of the reaction in some cases. The order of reactions has so certain defining features depending on this. In the case of a zero-order reaction, in which the rate of a reaction is independent of the concentrations of the chemical species involved in the reaction, the order of the reaction is zero.
- Important information to keep in mind is that the reactant concentration may slow down the reaction as well. That is, the concentration of the reactant has a negative relationship to the reaction rate. The order would therefore take on a negative value in that scenario.
- Furthermore, the order of reaction can take a positive value, with the rate being proportional to the concentration. When the amount of a reactant rises, the pace of the reaction quickens.
- In addition, the rate of a reaction is not always a positive or negative integer; in some circumstances, it may be a non-integer value. Quite a few complex reactions involve situations where the values involved are not integers.
How to Find Order of Reaction?
For every chemical reaction, the idea of the rate of reaction is very important. The order of reaction deals with the idea that how a reactant will influence the rate of a particular reaction. And by the knowledge of the order of reaction, we can focus more on the reactant that will be going to affect the rate of the chemical reaction. So, it is will not be obtained by simply looking into the chemical equation. It requires another method for finding the order of the reaction.
Following Methods Can be Used for Determination of Order of Reaction –
The order of reactions can be easily obtained by the following methods.
- From the rate equation: By using the rate equation of chemical reactions we can find out the order of the reaction. For example, the order of the following rate is, \(Rate = K{[A]^2}\) , and the order is two.
- By doubling reactants: Doubling the concentration of reactants present chemical equations and observing its effect can help to find out the order of reactions. The experimental method is always used for the determination of order.
- Differential method: The method is also called the initial rate method. As it involves the determination of the rate of reaction by altering one of the reactant concentrations while the rest of the reactant is kept constant.
- Graphical method: In this method, the chemical reaction that involves only one reactant is determined method. If A is the reactant involved, a graph is plotted in which logA vs time provides a straight line, it will be a first-order reaction. While the graph 1/A vs time is a straight line it will be second order.
- Integral method: The use of an integral form of rate law is used the determination the order of the reaction.
Difference Between Molecularity and Order of Reaction
Molecularity and order of reaction are two important terms that deal with chemical reactions. The difference between these two is tabulated below.
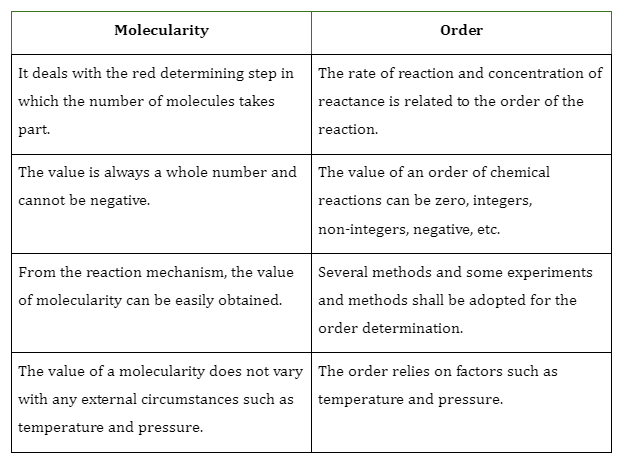
Zero Order Reaction
Zeroth order or Zero-order reactions are chemical reactions that don’t rely on the concentration of chemical species involved in the reaction. The exponents of the reactant in the rate equation are zero.
\(Rate = K{[reactant]^0} = K\)
An example of a reaction that involves a zero-order reaction is the decomposition of N2OThe reaction is,
First Order Reaction
The rate of reaction which counts on only one of the reactants is a first-order reaction. For such a reaction if the concentration of reactant is doubled the rate of the reaction will also get doubled.
The rate equation for such reaction is,
rate=k[A]
Hydrolysis of cisplatin, the drug used for the treatment of cancer is an example of a first-order reaction.
Second Order Reaction
The reaction in which the rate relies on the square of the concentration of reactant is a second-order reaction. For reactions,
2 A → products
The rate equation for the reaction will be,
\(Rate = K{[A]^2}\)
An example of such a reaction is the decomposition of Nitrogen dioxide,
\(2{\rm{ }}N{O_2} \to {\rm{ }}2{\rm{ }}NO{\rm{ }} + {O_2}\)
Pseudo First Order Reaction
Even though they occur at the second order, their behaviour is more like to that of the first. Such chemical reactions have a pace that is proportional to the product of two reactants, but the appearance of the third reactant cannot be predicted because its concentration will fluctuate so wildly. As a pseudo-first-order reaction, the hydrolysis of ethyl acetate provides a good illustration.
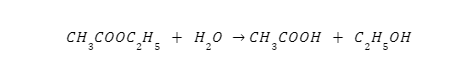
Summary
There is a correlation between the rates of chemical reactions and their respective orders of reaction. Understanding rates is crucial for the development of chemical processes. When calculating the order of a chemical reaction, the exponents included in the concentration data are added together. The reaction order has a unique property in that it can take on negative, non-integer, and positive values.
Reactions are categorised as zero order, first order, second order, pseudo-order, etc., depending on the sequence in which they occur. There are a number of strategies that can be used to establish the reaction’s order. Similarity order of reaction also includes the concept of molecularity, which is commonly used to describe chemical processes. However, these two concepts are not the same at all.
Frequently Asked Questions
1. How does temperature affect the rate of a chemical reaction?
Ans: The reaction rates increase with temperature. Arrhenius Equation is an empirical connection that models the fluctuation of the rate constant with temperature, revealing chemical reaction rates at different temperatures.
2. How is half-life used in radioactive dating?
Ans: Scientists use specialized equipment to measure the amount of an unstable isotope in a sample and the amount of the isotope that it becomes after the isotope decays.
3. Does molecular size affect reaction rate?
Ans: The larger and more complex the reactant molecules, the less chance there is of a collision at the reactive site. Does larger the molecular size of the reactant, lower will be the rate of reaction.

