Introduction
Mixing various compounds is a key aspect of Chemistry. In science, a mixture is a substance mixed with 2 or more relatively simple materials. These substances can be either elements or compounds. Compounds are unadulterated substances. They are composed of the same molecules. A compound’s molecules are made up of two or more different types of atoms that are chemically bonded together. Mixtures are composed of two or more substances — elements or compounds — that are physically but not chemically combined; they lack atomic bonds. Pure substances are elements and compounds that contain only one type of molecule. A mixture is made up of two or more different types of pure substances. In a mixture, the molecules of these substances do not form any chemical bonds. A mixture’s components retain their chemical independence while physically blending together. These components are frequently visible and distinguishable visually.
What is a Mixture?
A mixture would be a substance made up of 2 or even more components that have been physically mixed to maintain the characteristics of those constituents. In plenty of other terms, the properties of a mixture have been fully determined by the components that are present. We may divide the mixture into groups based on particle size as well as uniformity.
Types of Mixtures
Mixtures can often be divided into two types:
- Homogeneous Mixtures
- Heterogeneous Mixtures
Homogeneous Mixtures
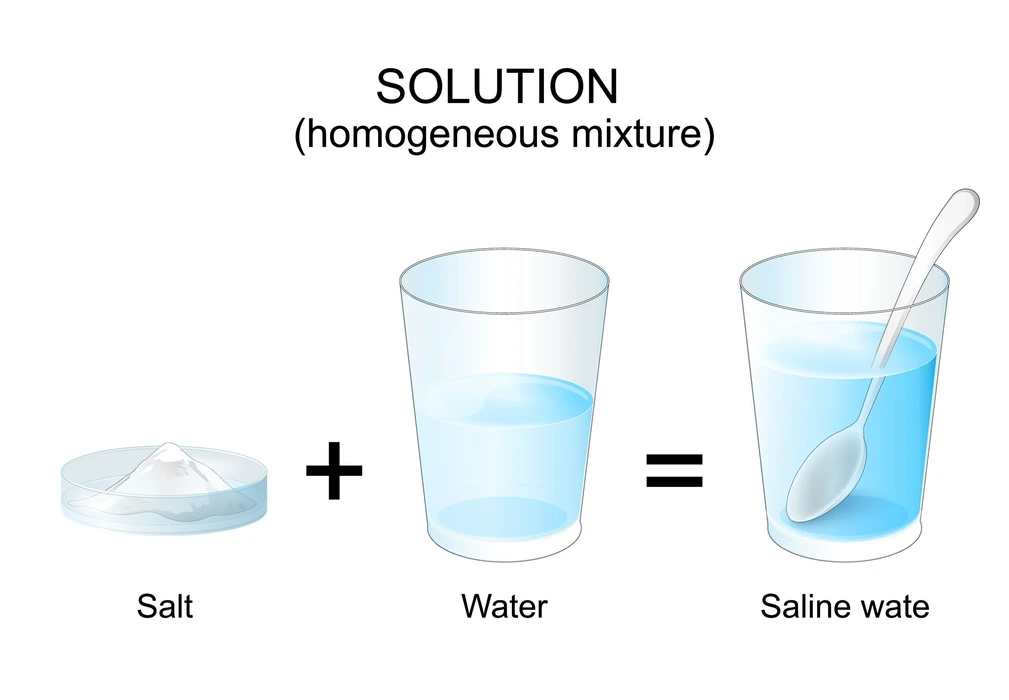
Homogeneous mixtures are those that have the same composition but also characteristics across their mass as well as body. Light does not flow via these elements. Sugar syrup, alcohol, as well as water are all homogeneous mixtures with particles of varying sizes that make identification difficult.
Heterogeneous Mixture
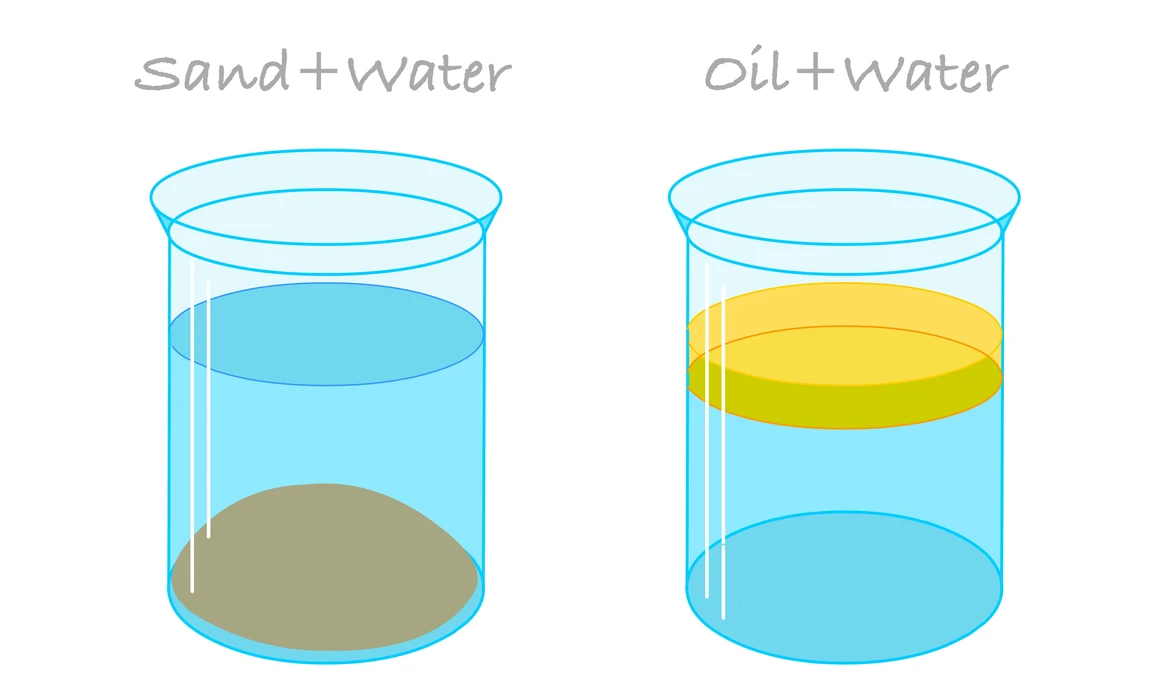
Heterogeneous mixtures include those mixtures that do not dissolve properly but also do not have similar content. Particular elements are frequently detectable and might even be isolated using both chemicals and physical properties due to such characteristics. Suspensions, as well as colloids, are often the 2 types of heterogeneous mixtures. For example, water and sand, blood, or starch.
What are Compounds
Compounds are atomic components as well as other elements that are linked collectively with a chemical bond. Depending on the substance, such a bond might be ionic, covalent, as well as metallic. Because all compounds possess a fixed ratio of components, they are uniform. Certain substances differ from elements that normally mix to form only one compound unit in terms of their characteristics. Furthermore, a chemically bonded molecule cannot ever be physically detached.
Types of Compounds
Compounds are classified into 3 types:
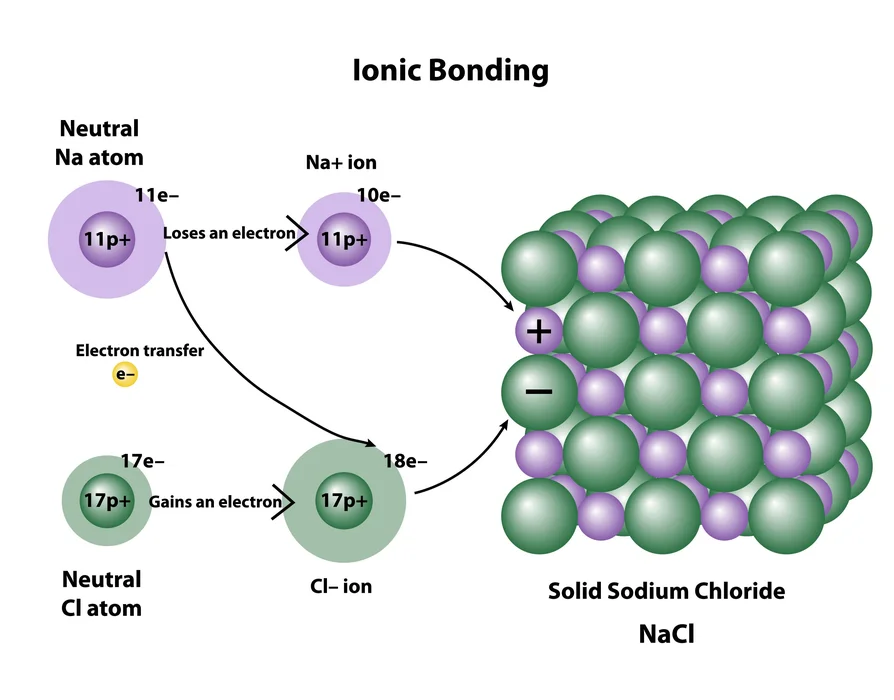
- Ionic compounds: They are made up of two oppositely charged ions. Electrostatic attraction holds the ions connected. Water is usually reactive in ionic compounds.
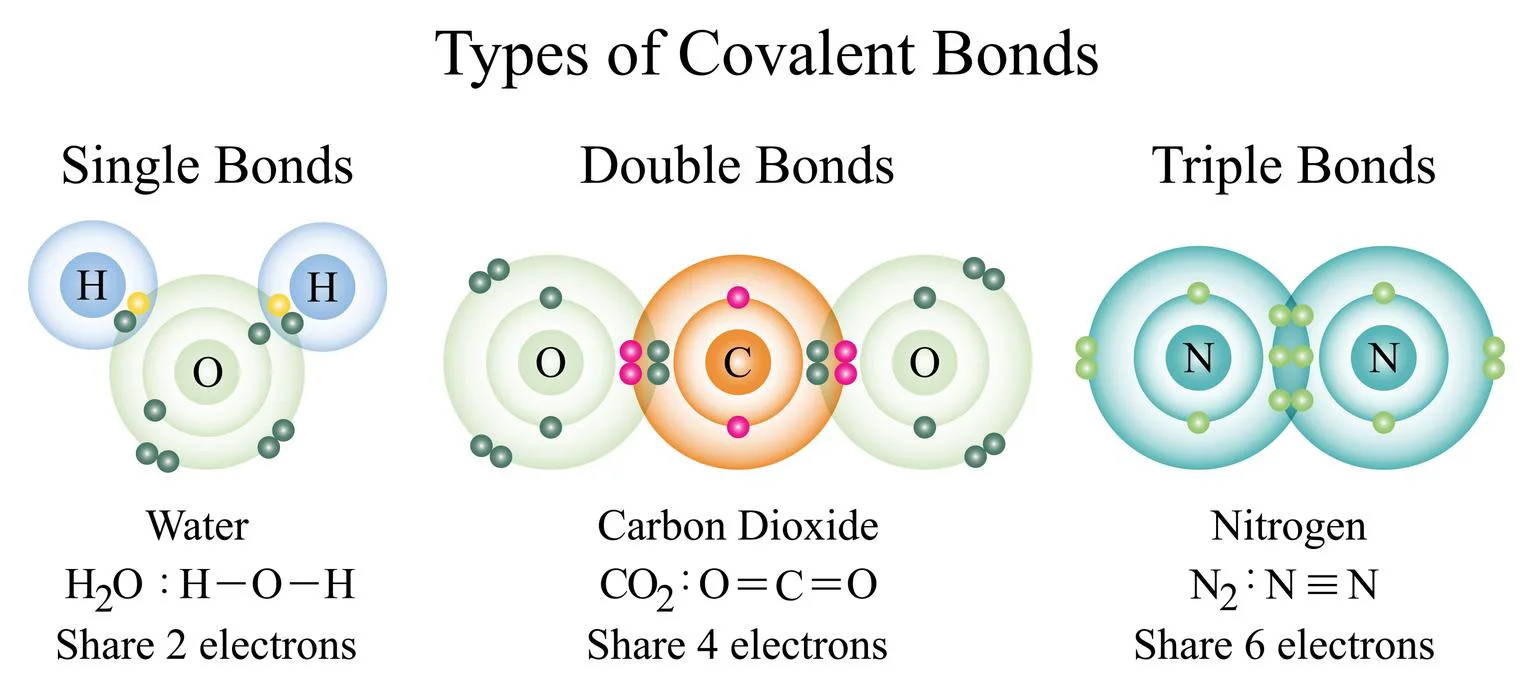
- Covalent compounds: They’re made up of atoms that exchange electrons and are also non-polar, which means they don’t even react with water.
Examples of Compounds
- Water: This is composed of 2 elements: 2 hydrogens as well as 1 oxygen.
- Methane: It is composed of 2 elements: carbon as well as hydrogen.
- Table salt: Sodium, as well as chlorine, are indeed the 2 elements found in table salt.
- Glucose: It is composed of 3 elements: carbon, hydrogen, as well as oxygen.
What are the differences between Mixtures and Compounds?
| Compounds | Mixtures |
| Chemical interaction between two or more components tends to produce compounds. | Mixtures are introduced by directly integrating two or more elements in such a way that no chemical reaction occurs between both components. |
| To yield a compound, elements must always join in a defined mass proportion. | The proportion of elements is not set or could change. |
| Throughout the development of a compound, its energy changes. | There is no change in energy. |
| It cannot be removed physically and must be separated using sophisticated scientific methods. | Physical separation of mixtures is possible. |
| The constituents’ properties are lost, or the compound generated has distinct physical as well as chemical properties. | A mixture’s constituents maintain its original properties. |
| Organic as well as inorganic compounds, both are possible. | Homogenous as well as heterogeneous mixtures can exist. |
| In compound initiation, new bonds have been generated. | There is no new bond forming. |
| The melting or boiling points of compounds are fixed. | The melting or boiling points of mixtures are not set. |
A mixture is formed by mechanically combining two or more components while retaining their distinct characteristics. It can exist as solutions, suspensions, or colloidal particles. Chemical components and compounds, for example, can be mechanically blended or mixed to form mixtures, but no chemical binding or another type of chemical transformation occurs, so each constituent retains its distinct chemical properties.
Frequently Asked Questions
1. What are the basic types of the mixture?
Ans. Two broad categories of mixtures are-
- Homogeneous mixtures
- Heterogeneous mixtures
2. Bronze is an alloy or mixture of which metals?
Ans. Bronze is a solid-solid mixture of copper(Cu) and Tin(Sn).
3. The solution is which type of mixture?
Ans. The solution is a homogeneous type of mixture where all the components or substances are uniformly distributed that cannot be separated manually or physically.