Introduction
The energy shift that occurs when an electron is added to an isolated atom is known as the electron gain enthalpy. Electron affinity is the capacity to accept an additional electron and produce an anion. Due to attaining a stable electronic structure, the elements going through this energy change or electron addition. In this process, an electron is added to chlorine to create a stable octet.
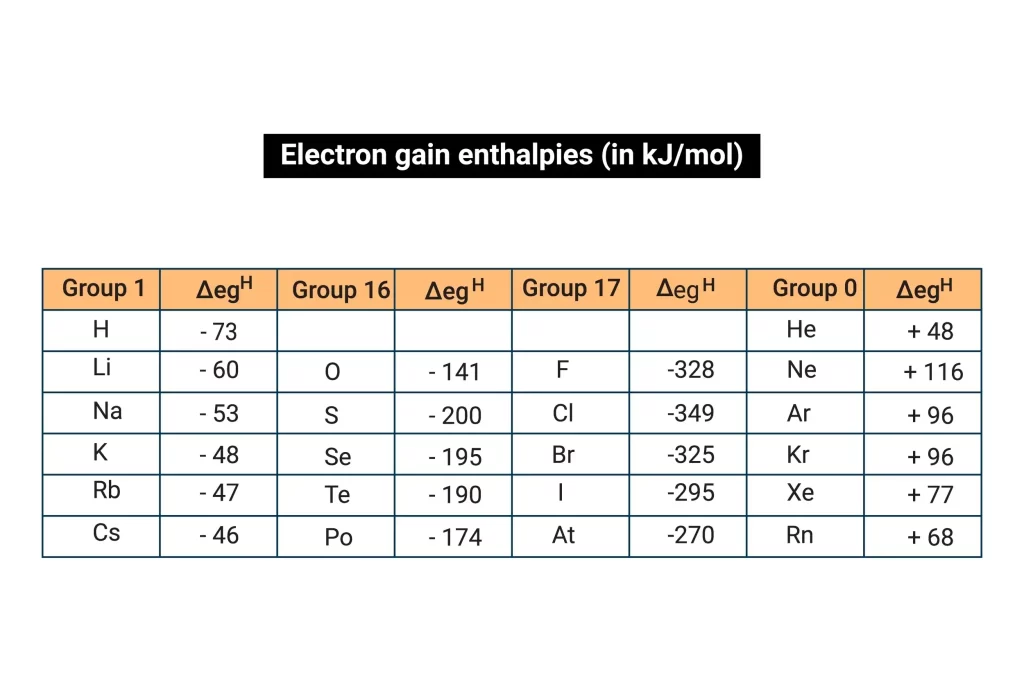
The groups 6th and 7th of the periodic table are where the majority of the electron affinities and negative ions or anions are found. In accordance with the size and nuclear charge of the elements, it can have either negative or positive values. For instance Sulphur has an electron affinity of -210 kJ/mol and chlorine has an electron affinity of -359 kJ/mol.
Sulphur and chlorine both release significant amounts of energy, but chlorine has a greater negative electron gain enthalpy. Sulphur, however, has less negative electron gain enthalpy than chlorine. This is so because although sulphur needs two electrons to form a noble gas structure, chlorine just needs one. Chlorine rapidly accepts an electron to gain stability, but compared to sulphur, which accepts two electrons, it loses more energy and turns more negative. Only when adding an electron requires a significant amount of energy do elements also have a +ve electron gain enthalpy.
What is Electron Gain Enthalpy of Elements?
The process of adding an electron to create an anion is known as the enthalpy or energy change. It refers to the energy that is emitted or absorbed when an electron is introduced to a gaseous atom in isolation. The symbol for it is egH. The quantity of energy released determines how much an element’s electron gain enthalpy increases. Chlorine has a larger negative electron gain enthalpy value compared to fluorine. This is due to the fact that chlorine’s outermost shell has a lot of room for newly added electrons or incoming electrons and releases the greatest energy.
Factors that Affect Electron Gain Enthalpy
The following list of variables affects an element’s electron gain enthalpy:
- When the atomic size increases, the electron gain enthalpy falls. When the distance between the nucleus and the outermost shell widens, the huge size of an atom reduces the force of attraction for the arriving electron at the nucleus.
- The stable configuration components contain subshells that are partially and fully filled. For those elements that require to add electrons to their outermost shell in order to attain maximal stability, the electron gain enthalpy will be larger.
- Nuclear charge has a direct impact on electron gain enthalpy. The overall positive charge that the entering electron experiences makes up the atom’s effective nuclear charge. The effective nuclear charge has increased.
Electron Gain Enthalpy in Period
The electron gain enthalpy rises in the period as we move from left to right. As we move from left to right in the period, the effective nuclear charge increases and the size of an element decreases.
Electron Gain Enthalpy Group
When an element’s size grows and its effective nuclear charge drops as we advance down the group, electron gain enthalpy reduces. The approaching electron will feel less attraction as a result. When we descend the group, electron gain enthalpy thus diminishes.
Measurement and Use of Electron Affinity
Electron affinity is a quantitative method to measure how easily an electron is added to a neutral atom, thereby forming a negatively charged ion and, hence releasing energy. It is applicable for gaseous atoms only, as solids and liquids state change their energy level due to contact with other molecules and atoms.
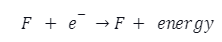
- The energy released during the chemical reaction or process is given as a negative number. Exothermic process is another name for it. Eea or EA is used to symbolise it. The unit is given in kJ/mol.
- As the amount of an element decreases in a group, electron affinities for those elements have negative values or become less negative since it takes more energy to add an electron, making \({E_{ea}}\) less negative.
- Electron affinity is used to measure the polarity of bonds and judge their ionic and covalent characters.
Electron Gain enthalpy
One-Electron Reduction
The gain of electrons or the addition of electrons to generate a negative charge on materials is known as the reduction process. It is claimed that the atom has been decreased by one electron.
Summary
The electron gain enthalpy is the amount of energy that is released when an additional electron is added to a neutral atom. In contrast, electron affinity is the tendency of an element to accept an additional electron and produce an anion. The energy released to achieve stability has a negative value when an electron or first electron is added. The electron gains enthalpy or a negative value as more energy is released. An anion’s value becomes less negative or positive as a result of the addition of a second electron because of the stronger repulsive forces that arise from having more electrons in the system.
Frequently Asked Questions
1. Comment on electron gain enthalpy of electropositive elements.
Electropositive elements have a tendency to lose electrons and form stable cations. As a result, adding one electron requires a lot of internal or external energy, hence their electron gain enthalpy will be positive.
2. Why is electron gain reaction exothermic?
When an electron is introduced to an isolated gaseous atom to create an anion, energy is released. The neutral atom attains a stable electronic configuration which results in release of energy.
3. What is the enthalpy of electron gain for group 18 elements?
The outermost shell of group 18 elements are completely occupied, and their electronic configurations are constant. When adding electrons demands a significant amount of energy, the electron gain enthalpy turns positive.