Introduction:
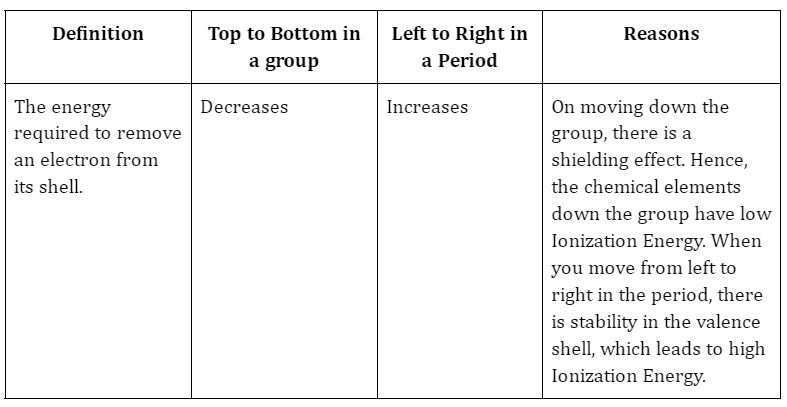
An alteration in an atom’s energy is known as electron affinity. An atom gains a negative charge and releases energy as more electrons are added to its outer shell. In order to stabilise its octet, an element obtains electrons. When an element receives or loses an electron, energy is released. An exothermic reaction occurs when an element takes an electron to form a compound, releasing energy in the process. An exothermic process releases energy because a nucleus from another element is using it to attract the electron. An endothermic process is one in which energy is absorbed when an element loses an electron. An atom gets energy when one or more of its electrons are lost.
What is electron Affinity?
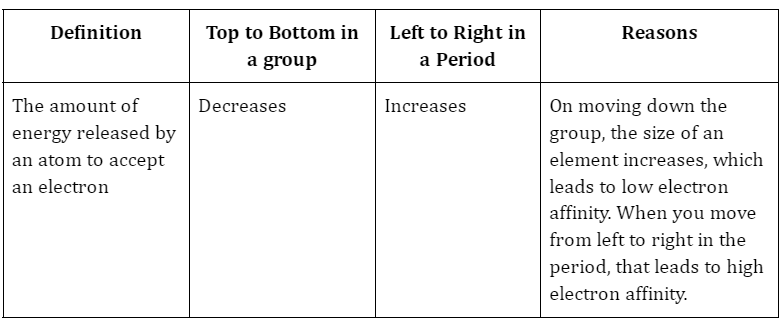
A molecule either loses or gains energy during a chemical reaction. Energy is gained or lost as electrons are gained or lost. Exothermic reactions occur when an atom loses an electron, releasing energy. Chemical reactions in which electrons are lost are called endothermic because they consume energy. The ability to accept an electron is referred to as the electron’s affinity. when a neutral gaseous atom accepts the electron, it will charge with a negative ion. There is always a negative value for the first electron affinity and a positive value for the second. Atomic electron affinity measurements are notoriously imprecise. Ionic compounds’ energy dissipation is used as a proxy for this. The propensity of an atom to act as an oxidising or reducing agent is another indicator of its electron affinity. Kilojoules per mole is the unit of measure. Ea represents the attraction between electrons.
First Electron Affinity:
When an electron is added to an atom that is electrically neutral, the atom gains a negative charge and releases energy. Since greater energy is needed to draw an electron via the nucleus, the initial electron affinity is usually negative.
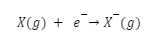
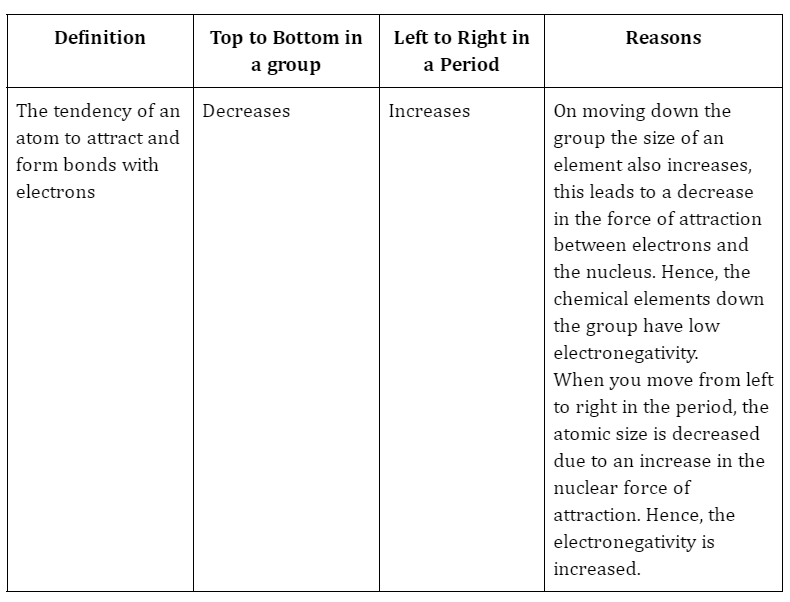
The common trends followed in the periodic table are:
- When one moves down the periodic table, electron affinity decreases.
- The electron affinity rises as we progress from left to right over the time.
- When metals lose an electron, they become cations.
- When a nonmetal accepts an electron, it forms an anion.
- Due to the ease with which they may lose an electron from their outer shell, metals have a lower electron affinity than nonmetals.
- It is an endothermic process for metals to lose one electron because the outer shell electrons have less attraction. An Element of Matter
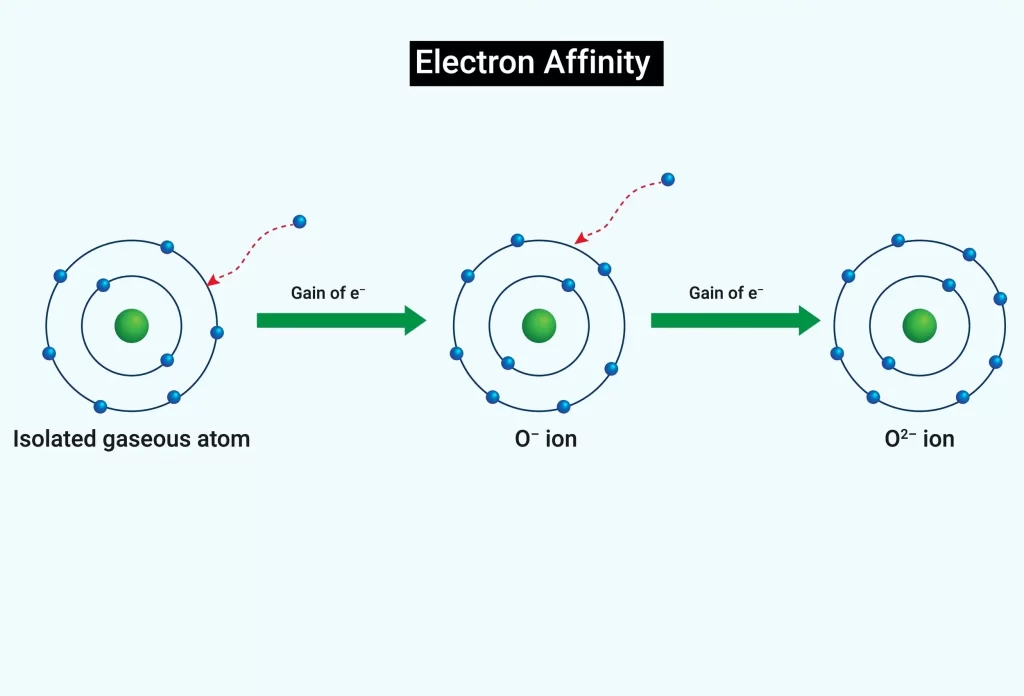
Electron Affinity of atom
Second Electron Affinity:
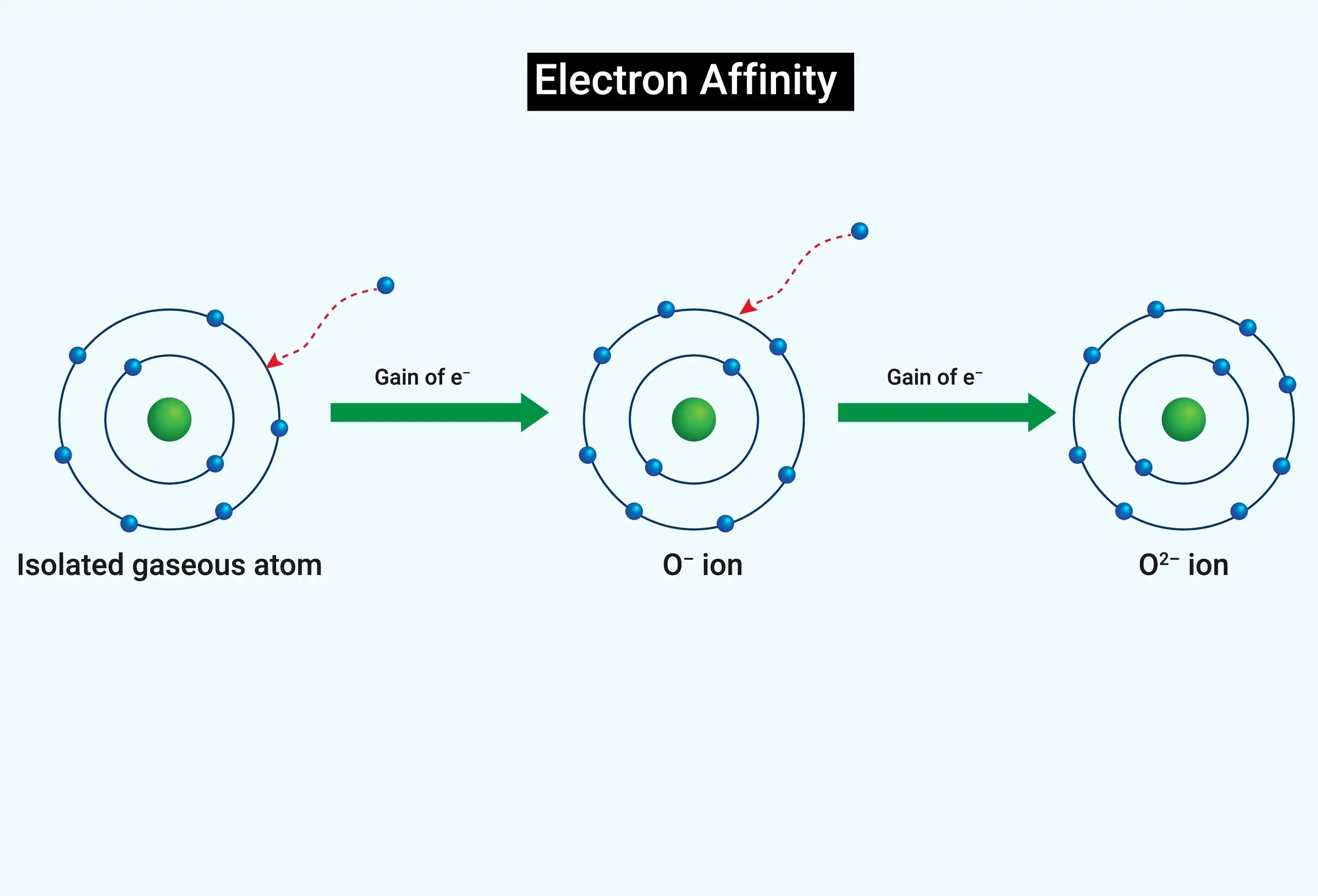
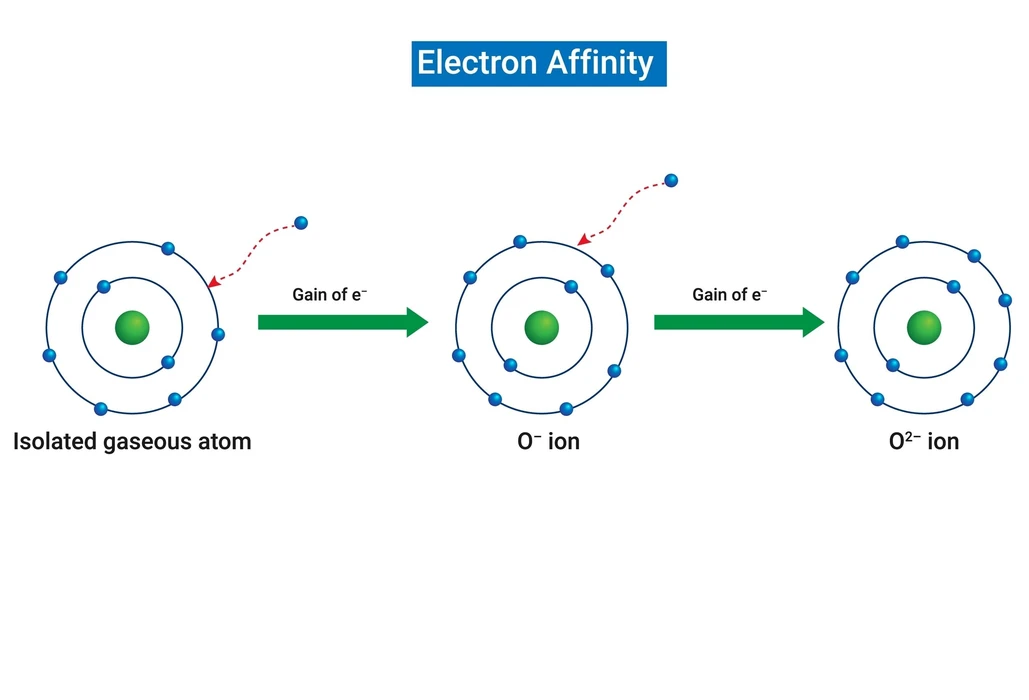
Gaining an electron is the anion’s second electron affinity. Second electron affinity manifests itself in the group-16 elements oxygen, sulphur, and selenium because these elements form anions with (-2) ions.
For example:
The electron affinity of oxygen is given as;
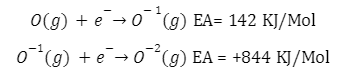
The second electron affinity is higher than the first electron affinity in oxygen molecules because of the electron-electron repulsion in the negatively charged ion of oxygen.
Factors Affecting Electron Affinity:
The electrical arrangement of atoms, the nuclear charge of the molecules, and the atomic size all play a role in determining a molecule’s electron affinity.
- Atomic size and its effect: The electron affinity of smaller atoms is higher than that of larger ones. The nucleus of a smaller atom is more alluring to the electrons than the nucleus of a larger atom. Attraction for electrons in the outer shell will diminish as atom size grows because the outer shell will be further from the nucleus. For instance, Br has a lower electron affinity than I.
- The electron affinity is also affected by the nuclear charge. A higher atomic charge results in a stronger electron attraction, and hence a higher electron affinity. When a molecule is already charged, the repulsion between its electrons and the pull from the nucleus both increase, leading to a lower electron affinity in the charged ion.
- Reducing the screening effect on the inner shell of an atom increases its electron affinity.
- The electron affinity is also affected by the electrical configuration. Inert gases will have zero electron affinity because elements with a complete octet have no propensity to receive electrons. An important factor in electron affinity is the electrical configuration. Because of their unique electrical structure, metals have a lower electron affinity compared to non-metals.
Summary
The capacity to take electrons in a gaseous state and transform into an anion is known as electron affinity. The process of receiving electrons is exothermic because it results in the release of energy. The electron affinity reduces when we travel up to down in groups and rises while moving left to right in a period. It is denoted by the symbol Ea, and it is measured in kilojoule per mole (KJ/Mol). In all cases, the electron-electron repulsion causes the first electron affinity to be lower than the second electron affinity. The electronic configuration, screening effect, and nuclear charge of elements all have a role in how strongly they attract electrons.
Frequently Asked Questions
1. Why does fluorine have less electron affinity as compared to Chlorine?
As we move along a period, electron affinity increases and decreases down a group. However, the fluorine atom is too tiny to release a significant quantity of energy. Hence, among the halogens, chlorine has the highest electron affinity value, followed by fluorine, bromine, and iodine.
2. Group 1 or group 7—which is more reactive in the periodic table?
Group 7’s nonmetals, the halogens, become progressively less reactive as you move from top to bottom of the group. This pattern runs counter to what we observe in Group 1 of the periodic table, which contains the alkali metals.
3. What is the sign of electron affinity?
Adding an electron to an element makes it less positive, hence the electron affinity is negative at initially. Nevertheless, adding an electron to a negative ion might make it more positive or more negative depending on the nature of the repulsion between the electrons.