Introduction
One type of electrochemical cell is the electrolytic cell (EC), which converts electrical energy into chemical energy. This process causes an artificial redox reaction. Electrolytic cells may break down a wide variety of chemicals. It’s a technique for getting rid of ions in a mixture. The reduction half-cell and the oxidation half-cell together make up a single electrolytic cell. Energy for voltaic cells (VC) comes from a chemical reaction that happens naturally and results in a flow of electrons across a circuit (external). These cells are essential because they form the backbone of batteries, which provide the energy that runs modern civilization. Electricity is typically used to fuel non-spontaneous processes.
About Electrolytic Cell
It’s a device for electrolyzing a chemical compound, or breaking it down using an electric current. Simply put, this is a specific kind of electrochemical cell. Hydrogen (\({H_2}\)) and oxygen (\({O_2}\)) are produced when water is electrolyzed. Here, a bit of more power is required to get beyond the threshold energy level.
The electrolyte in these cells is often a fused or disintegrating ionic compound, and the cell itself consists of two electronic or metallic conductors (electrodes) that are either separated from one another or remain in touch with one another.
One of these is the use of a direct electric current source to link these electrodes, which keeps one of them negatively (-vely) charged while leaving the other, maybe positively (+vely), charged.
The cathode (-ve) moves the anode (+ve) and transfers one or more electrons to the cathode. This creates brand-new particles, either neutral or ions. Electron transmission is the determining factor in the cumulative effect of these two processes.
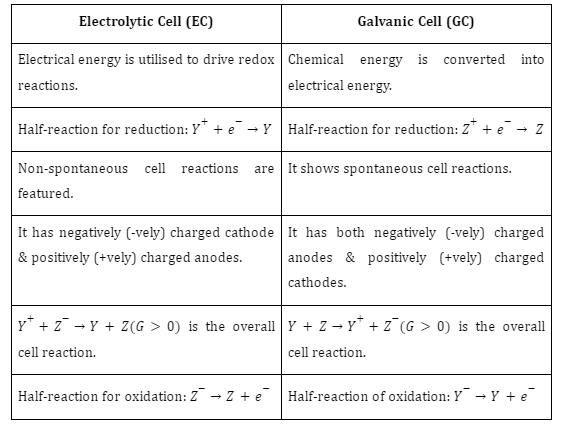
Galvanic Cell and Electrolytic Cell Comparison
Both electrolytic and galvanic cells function in the same way. There are three main features that make electrolytic cells and galvanic cells similar: Both cells need a salt bridge for proper functioning. Electrons (e-) always flow from the anode to the cathode, and both have cathode and anode components.
Difference Between Galvanic Cell and Electrolytic Cell
Electrolytic Cell Application
Some of the applications of electrolytic cell are
- Generation of oxygen gas from water .
- Electroplating
- Electrorefining.
- Electrowinning
- Isolation of metals of commercial importance such as aluminium, gold, copper, zinc, etc.
Properties of Galvanic and Electrolytic Cells
Galvanic cells (GCs) are devices used to convert chemical energy into electrical energy. Electrolytic cells are capable of converting electrical energy into chemical energy. A salt bridge unites two segments housed in separate containers. The oxidation reaction takes place at the anode, and the redox reaction at the cathode. Electrons (e-) are supplied by an external cell and flow from the cathode to the anode.
Working Principle of an Electrolytic Cell
Electrolytic cells are instruments based on the idea that during an oxidation-reduction reaction, electrons (e-) are transferred from one chemical species to another.
Lets take the example of electrolysis of NaCl.
Sodium metal and chlorine gas are produced during the decomposition of molten sodium chloride (NaCl).
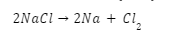
Electrolysis occurs when a second energy source is applied to a vessel containing molten sodium chloride and carbon (C) electrodes. At the cathode end, the Na+ ion will be neutralised by the flow of electrons (e-) from the anode.
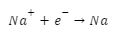
Although the anode and cathode retain their traditional roles, oxidation and reduction now occur at the cathode and anode, respectively. The electrolyte cell’s operational parameters are crucial to its proper functioning. Even the most potent reductant will succumb to oxidation. The most oxidising substance will be the one to be depleted.
The success of the Electrolytic Cell Molten sodium chloride (NaCl) depends on the electrolysis process, which is an integral part of the electrolytic cell.
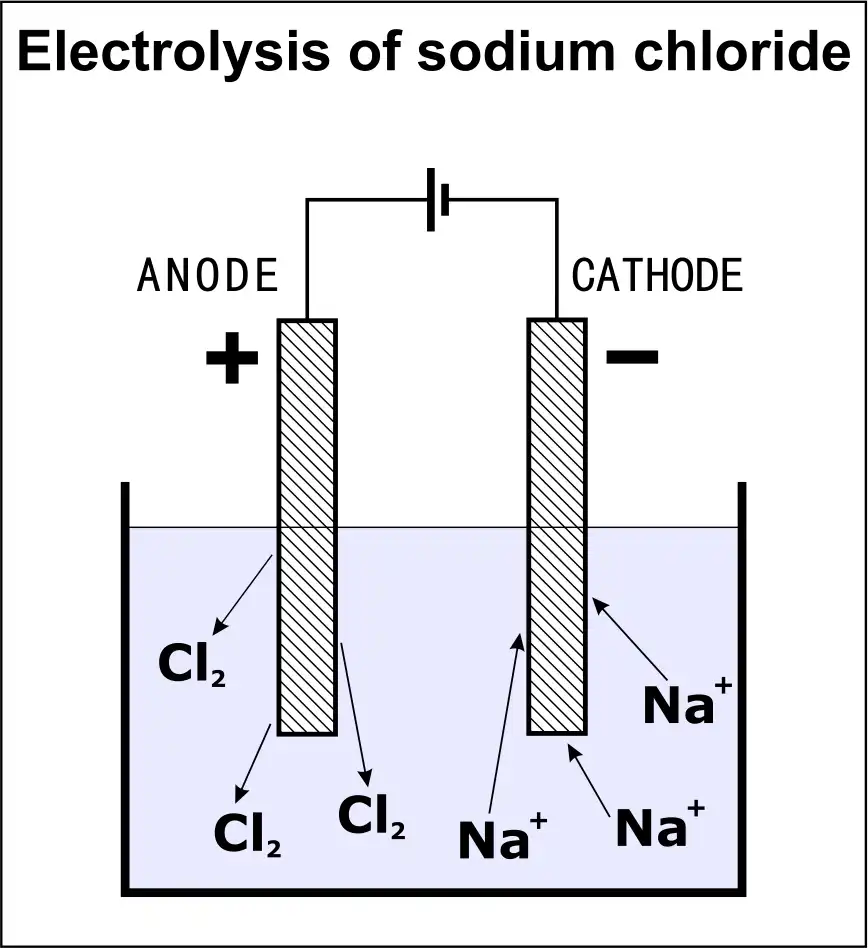
Electrolysis of NaCl
Two non-active electrodes are submerged in a bath of liquid NaCl. Dissociated Na+ cations and Cl- anions are present here. The cathode is the part of the circuit where electrons (e-) gather when an electric current is sent through it. As a result, the resulting charge is negative (-ve). Because of this, the positively charged sodium (Na) cations go to the cathode, which is negatively charged. This results in the formation of sodium metal (Na) at the cathode.
Chlorine Cl atoms are transferred to the cathode during this process. It causes chlorine gas to be generated at the anode (Cl2). Two electrons (e-) are then freed, completing the circuit. In an electrolytic cell, molten NaCl is electrolyzed to produce Na and Cl2 gas, two metals (EC).
Summary
An electrolytic cell (EC) is a device that converts electrical energy into chemical energy via a non-spontaneous redox action. A reduction half-cell and an oxidation half-cell make them up. An electrolytic cell consists of the cathode, anode, and electrolyte. Similarities and differences exist between electrolytic cells and galvanic cells. As an example, the electron (e-) in each cell is unique. Understanding electrolysis requires familiarity with Faraday’s laws. The magnitudes of electrolytic effects are defined by them.
Frequently Asked Question
1. Define EMF?
Maximum potential difference between a cell’s electrodes is its electromotive force (EMF). The net voltage between the oxidation and reduction halves of a process is another way to think about it. The electromotive force (EMF) of a cell is the primary criterion for establishing the galvanic nature of an electrochemical cell.
2. What is the meaning of zero EMF in a battery?
After the chemical process within a battery is complete, the anode and cathode have the same number of electrons, and the electrical field between them is zero. When a reverse field is applied to a rechargeable battery’s contacts, the battery’s chemical makeup is returned to its previous state, allowing it to once again discharge electrons in an electrical circuit.
3. What is the relation between the current and the electrolytic product?
The 1st law of Faraday says that the amount of electric current flowing through an electrolyte causes the same amount of chemicals to build up as the amount of current. Faraday’s law of electrolysis says that when the same amount of electric current flows through many electrolytes, the amount of materials that build up is proportional to their chemical equivalent.