Introduction
Protein, DNA, and RNA can all be separated from one another using an external electric field and this laboratory technique. As the gel or matrix employed includes a given size and enables just a particular molecule, the separation of molecules occurs on the basis of charge and size. Only very small particles, such as atoms or ions, may fit through the pores. By doing so, molecular separation is possible.
When electricity flows, ions can migrate to various poles. In electrophoresis, the movement of anions is known as anaphoresis and that of cations is known as cataphoresis. When a current is applied, proteins migrate to the positive poles, where they may be more easily separated.
What is Electrophoresis?
Electrophoresis is the process by which a current drives charged molecules to separate poles. Molecules with a positive charge will go away from the cathode and towards the anode. Negatively charged molecules will seek out a cathode, which is the opposite pole of an electrode.
As proteins and nucleic acids are negatively charged, they gravitate towards the cathode. Because to its association with electricity and ion motion, electro kinetics is another name for this field. Capillary electrophoresis and slab electrophoresis are the two most common forms. Gel and paper electrophoresis are examples of capillary electrophoresis, while Iso-electro focusing and Zone electrophoresis are examples of slab electrophoresis.
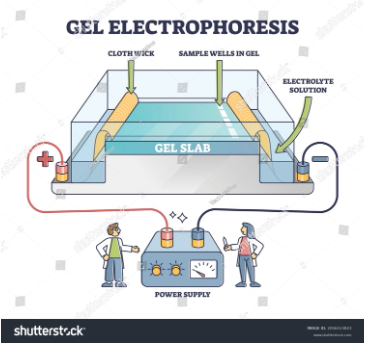
Gel Electrophoresis
Applications of Electrophoresis
Electrophoresis is applied in many fields because of its feasibility and inexpensive mode. Some of its applications are,
DNA Fragmentation and DNA Analysis
Gel electrophoresis is the process used for the separation of DNA fragments based on their size. The approximate size of DNA is also obtained in this process
Protein Detection
Gel electrophoresis is again employed for the detection of proteins. Proteins have higher migratory power and that is employed for its detection. By the application of external current, the charged particles of proteins will migrate towards their opposite electrodes. As protein is negatively charged it will migrate towards the cathode. It can also use the liquid matrix for the separation. The majority of protein applications can be achieved by the use of this process. They include purity determination and purification of proteins.
Testing of Antibodies
Immune electrophoresis detects antibodies. Antibodies on a gel do it. blood test. If the sample contains the antigen, an antigen-antibody complex will form. IgA, IgM, etc. are easily diagnosed.
Factors affecting the Process of Electrophoresis
Some factors can substantially affect the process of electrophoresis. They are,
Electric Field: Electric field is responsible for the movement of charged particles towards the oppositely charged electrodes. So it plays an important role in the process of electrophoresis.
Sample features/details: The features of the sample such as size, shape, and charge have an important role since it is a size-based and charge-based separation. If the charge of a molecule is high the rate of migration will also high. But if the size increases the rate will decrease.
Buffer: For stabilizing the pH of the medium buffer solution is necessary the use of zwitterionic buffers will be much better.
Supporting Medium: It plays a predominant role since the medium can alter the rate of migration by having some adsorption property. Electro osmosis will also result due to some mediums. So selection of a proper support medium will increase the rate of migration.
How does gel electrophoresis work?
Gel electrophoresis is a type of electrophoresis in which charged particles or ions of biological molecules can be separated by the application of an external field. They move through a gel hence the name gel electrophoresis is used. And its movement is called migration. And the migration of ions is to oppositely charged electrodes. The electric current for this process is applied across the gel. The gel acts as a sieve since it contains small pores that can only allow small-sized molecules. DNA fragments are distinguished using this electrophoresis. And DNA is a negatively charged species so it will migrate to the rds cathode.
What is agarose gel electrophoresis?
One of agar’s main components is a matrix for the electric current-based synthesis of macromolecules. Agarose gel is commonly employed as a matrix in the separation of DNA and big protein molecules because it is simple to cast, has a good pore size, and has a good gel strength. It has several charged groups and aids in water movement in the opposite direction of DNA movement towards the anode. A larger concentration of agarose gel is required for the separation of tiny molecules.
What are the advantages of electrophoresis?
The advantages of electrophoresis are,
- The mode of operation of this technique is easy.
- Short time is only needed.
- Needs only a low sample.
- The consumption of electrolytes during the process is low.
- Can be used as an analytical tool and also as a synthesis tool.
- The bulk amount of proteins shall be separated.
- Inexpensive apparatus.
Summary
Samples are separated by size and charge using electrophoresis. It is medium dependent, and the various forms it takes are defined by the medium used. Several types of electrophoresis in tubes and capillaries. Gel electrophoresis, zone electrophoresis, etc., are also included. Gel electrophoresis stands out because it is used to separate proteins, DNA, RNA, and other biomolecules. Gels aid the separation process since only molecules of a certain size may pass through them; if an electric field is applied, the molecules in question will align themselves into a distinct band, making them easy to identify. The supercoiled structure of agarose gel makes it a viable alternative matrix. Electrophoresis has various benefits due to being a cheap and efficient method.
Frequently Asked Questions
1. What is the difference between SDS-PAGE and Native PAGE?
Ans. SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) is a technique used to separate proteins based on their size and charge. Native PAGE (polyacrylamide gel electrophoresis) is a technique used to separate proteins based on their size and shape.
2. What is Capillary Electrophoretic Enzyme Assay?
Ans. Applications of the analytical separative technique known as capillary electrophoresis (CE) extend far beyond the realm of enzymatic research.
In an open capillary, the substrate and the product are separated by size and charge in an electric field.
3. What is the haemoglobin electrophoresis test?
Ans: It is an electrophoresis test that can be used to the identification of the amount of haemoglobin present in the sample blood.