Introduction
Among the several intermolecular forces that exist, Van der Waals forces are notable. Johannes Diderik van der Waals, a Dutch scientist, proposed them in 1873 and so they bear his name. When compared to other intermolecular forces like hydrogen bonding and ionic bonding, Van der Waals forces are weak. Nonetheless, they continue to play a significant role in numerous branches of chemistry and physics.
In contrast to covalent and ionic bonding, which are both based on shared electrostatic repulsion between atoms, weak interactions are facilitated by correlations between the wildly varying polarisations of neighbouring particles (a consequence of quantum dynamics).
What are Van Der Waals forces?
Van Der Waals forces refer to the attractive and repulsive interactions that act between molecules and between atoms. The polarisation variations of neighbouring particles create these bonds, setting them apart from covalent and ionic interactions.
As a result of transient dipoles formed by the unequal distribution of electrons, molecules are attracted to one another via Van der Waals forces. This may occur due to the proximity of two molecules or the presence of a persistent dipole in one of the molecules. Because of the dipoles, the molecules are drawn to each other via a van der Waals force.
Characteristics
- Covalent bonds involve electron sharing, while ionic bonds require one or both atoms to give up an electron. They’re both attracted to each other with a force that’s orders of magnitude stronger than Van Der Waals’.
- Multiple independent interactions compose them, making them cumulative.
- These forces do not have a direction and are thus impossible to fully exhaust.
- They do not vary with variations in temperature. This is because the amplitude of these forces is greatest when the interacting molecules or atoms are in close proximity to one another, and they only act across a short distance.
Types of Van Der Waals Forces
London Dispersion Forces
When the electrons in two neighbouring atoms are in locations that cause the atoms to form temporary dipoles, an attractive attraction known as the London dispersion force is produced. An induced dipole-induced dipole attraction is another name for this force.
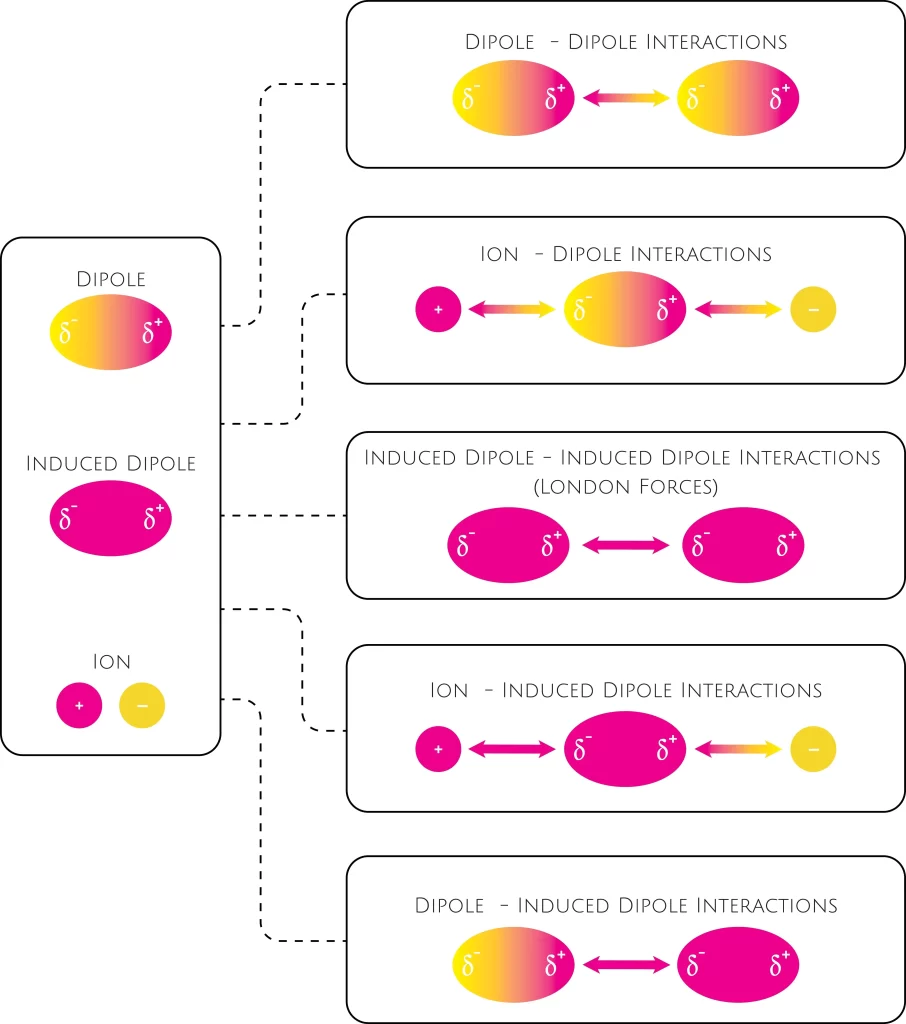
Dipole-Dipole interaction
Two polar molecules attract one another due to the attraction forces of their constant dipoles. These dipoles are formed because of the disparities in electronegativity between neighbouring atoms.
Hydrogen Bonds
These are unique dipole-induced dipole interactions between hydrogen atoms and highly electronegative atoms such as oxygen, nitrogen and fluorine.
Debye forces
These forces emerge when attractive Coulomb forces between permanent dipoles are outweighed by the strength of interactions between the permanent dipoles and other atoms/molecules.
Factors affecting Van Der Waals forces
Nature of element
The nature of an element or a non-metal is determined by the strength of its Van Der Waals forces. Elements or non-metals found in a liquid or gaseous state rely on these forces, whereas some metals use cohesive forces.
Electron count in an atom/molecule
In a periodic table, the atomic radius and the number of electrons held by each nucleus both grow as one descends from group to group There are more opportunities for transient dipoles to occur when the number of electrons is high. When there are many dipoles in a solution, the Van Der Waals force between them becomes greater.
Shape of molecule
The chemical structure of a molecule—whether it is branched or unbranched—can affect the strength of intermolecular forces, which in turn affects boiling points.
Size of an atom
Attractive bonds have different strengths depending on the sizes of the atoms involved. The intermolecular interactions between atoms strengthen as the size of an atom grows.
Shape of an atom
The strength of an atom’s intermolecular forces depends on the shape of its molecules. Thin molecules have more potential to develop temporary dipoles than short, fat ones.
Applications of Van Der Waals forces
- Van Der Waals forces aid in protein folding and further solidify the protein in its final structure.
- They also facilitate the bonding of graphenes within graphite by acting as lubricants.
- Research and development in fields like supramolecular chemistry, nanotechnology, and polymer synthesis.
- For the most part, they are responsible for keeping the inert gases in a solid or liquid form.
- Due to the attracting force exerted at the ends of their feet, geckos can quickly and easily scale smooth surfaces.
- Spiders are similar in structure.
Summary
Molecules are attracted to one another by forces known as van der Waals forces. The two most common kinds of van der Waals forces are the weak London Dispersion Interactions and the larger dipole-dipole forces. They are influenced by many different things, such as the elements themselves, the molecular and atomic structures they are made of, and the sizes and shapes of their constituent parts.
Frequently asked questions
1. How do Van de Waal forces affect the viscosity of a substance?
Ans. Van de Waal forces can increase the viscosity of a substance by increasing the attraction between molecules, which makes it more difficult for them to move past each other.
2. Write the equation of Van Der Waals forces.
Ans. (P+n2a/V2) (V-nb) = nRT
The above equation demonstrates the main two kinds of properties present – the volume of both elements and the attractive forces between them.
3. What is the use of the Van Der Waals equation?
Ans. The Van Der Waals equation is helpful in calculating an actual value in the case of non-ideal gases.

