Introduction
We need the purest form of metalloids like silicon, and germanium for the production of semiconductors. These metalloids behave like metals as well as non-metals whenever necessary. They are extensively used all over the world in all electronic gadgets and appliances. U.S. scientist W.G.P fann invented this technology in the 1950s with a motive to purify these elements to their best. This process is also known as zone melting or travelling melting zone.
Define zone refining
- It is an advanced technique that combines crystallization and melting processes to isolate the cleanest crystals from contaminated components, specifically metals. The crystal is heated just enough during this process to melt any impurities, which creates a covering of molten zones that moves along the crystal’s surfaces.
- It is often used to describe the process of purifying a crystal that involves melting a small portion of the crystal. The produced molten zone is moved over the crystal.
- Impurities mixed with metals get melted at the forward border. The process leaves behind remains of the pure element that has crystallized.
Principle of zone refining
- This method of refining is based on the idea that impure substances are more soluble when they are molten. Every time a molten metal crystallizes during the cooling phase, impurities are quickly eliminated because they do not contribute to the composition of the pure crystals.
- By passing the liquid region through a rolling heater, contaminants are removed, and the resulting re-crystallized pure metals are left behind in the form of solid metal. The zone must travel as slowly as possible throughout this progressive process to produce a highly pure version of the metal.
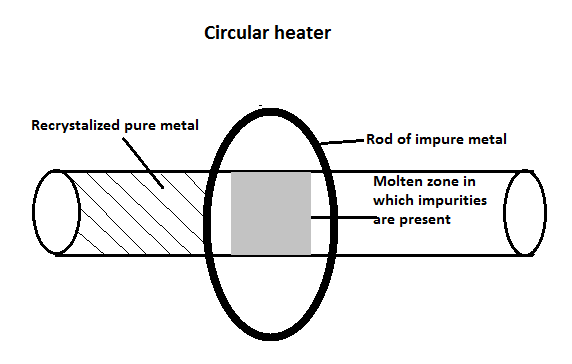
The Process of zone refining
- On one end of the contaminated metal pole that needs to be refined in the zone purification process, a movable radiator is installed. A portable heater in the shape of a circle is used to secure the unrefined metal rods inside a space that is filled with inert gas.
- This round heater heats the pole uniformly, producing a zone where the temperature steadily rises to the melting point in a direction perpendicular to the center of the rod. As the heater moves throughout the pole, the melted zone slides down the rod.
- Gradually warming the radiator all the impurities along with the pure metals liquefy, but molten impurities get transferred with the liquid zone whereas purified metal recrystallizes.
- Toward the completion of the cycle, solid purified metal stays on one side while contaminants build on the other. This process is repeated numerous times to get the purest form of the metal.
Application of zone refining
- To get the purest kind of metal, the zone refining process is typically used.
- Before creating an aqueous phase, this procedure is applied to concentrate biological components that are heat-labile.
- It is used to condense any enzyme, drug, protein, or other temperature-sensitive molecule during the crystallization process.
- Zone refining is a process used to make solar cells.
- It works remarkably well in cleaning impurities from semiconductors.
- Making organic compound benchmarks for High-Performance Column Chromatography, Fluorescence Spectroscopy, or even Spectrophotometry is a breeze with it.
- This process is very helpful for all analytical procedures that require the highest degree of purity for standardization or equipment calibration.
Limitations of the zone refining process
- Zone refining is a very costly procedure.
- Its applications are restricted to important synthetics and laboratory reagents.
- Zone refining must be utilized in conjunction with other processes in order to attain a sufficient level of purity.
Summary
Zone refining is a process used to separate the finest crystals from impurities. In this process, melting and crystallization methods are used. It works well for getting highly pure metals. Other names for it include zone melting, zone floating, and travelling melting zone method. It is a very expensive method that occasionally requires the use of additional methods to remove impurities. With this technique, impurities in semiconductor materials like silicon, germanium, and gallium can be successfully eliminated.
Frequently Asked Questions
1. Describe Vapour-Phase Refining
Ans: In the vapor phase refining process, the metal element should generate a volatile complex whenever a reagent is present. The complex must rapidly dissolve for the metal to be recovered. This causes the metal volatile complex to form, and this is the resulting complex that is broken down to produce pure metal. Nickel and titanium are such examples.
2. What is the process of distillation in the Purification of Metals?
Ans: Metals having boiling points are the only ones that can be purified using this procedure. It happens whenever a metal is heated above its boiling point, causing vapors to form. Since the vapors only contain pure metal that has condensed, the impurities are set aside before the vapors are transferred to another storage facility. For example mercury, and zinc.
3. What is the procedure involved in the electrolytic refining of metals?
Ans: The contaminated metal should act as the anode in electrolysis, losing ions continuously, while the pure metal acts as the cathode, receiving ions across the whole process. The metal with the smallest basicity is transferred to the anode when an electric current flows through this electrolytic mixture, whereas the metal with the maximum basicity stays in the mixture. For instance, copper and aluminium.