Introduction
There is matter in our universe. The matter is anything that maintains a quantity and a space. These things have a fundamental unit that cannot be divided into other parts with various chemical and physical properties. An atom is this fundamental component. An element is a substance that only contains one type of atom. Therefore, the species made up of a specific atom are the elements. For instance, there is only one type of atom in pure platinum metal. The atom was once thought of as an indivisible unit, but now it can be divided, releasing a huge amount of energy in the process.
Define an Atom and Molecule
An atom is the tiniest component of matter. The physical and chemical characteristics of the atoms that make up an element are all the same type. An atom is mono-nuclear, meaning that it has just one nucleus, which is surrounded by electrons and houses protons inside the central mass of the atom, the nucleus.
Chemical bonds bind the minimum required number of atoms in a molecule together. It is the joining of various atoms with the assistance of a chemical bond. The molecule oxygen\(\;({O_2})\) is a diatomic homo nuclear structure made up of two oxygen atoms bound together by a covalent bond.
What is the size of an Atom?
Only an estimation of an atom’s size can be made because it is impossible to measure it precisely. However, an atom’s atomic radius determines its size. Atomic radius is calculated by dividing the distance between adjacent atoms in a compound by two. Radii come in a variety of forms, including metallic, covalent, and ionic radii. The metallic radius is the separation between adjacent atoms in a metal. The covalent radius is the separation between adjacent atoms in a covalent compound. Ionic radii are the distances between adjacent ions in an ionic compound.
How atoms are formed?
The atom is the smallest unit of matter, consisting of a nucleus and electrons. The nucleus is the central portion of the atom that contains the positively charged proton and neutral neutron. And is surrounded by electrons that are negatively charged. Protons, electrons, and neutrons make up an atom. They are collectively known as subatomic particles.
Forces between Atom and a Molecule
Molecules are formed when atoms are held together by a strong chemical bond. These bonds are formed by the interaction of an element’s valence electrons to complete the octet. Chemical bonds are classified into several types. They do,
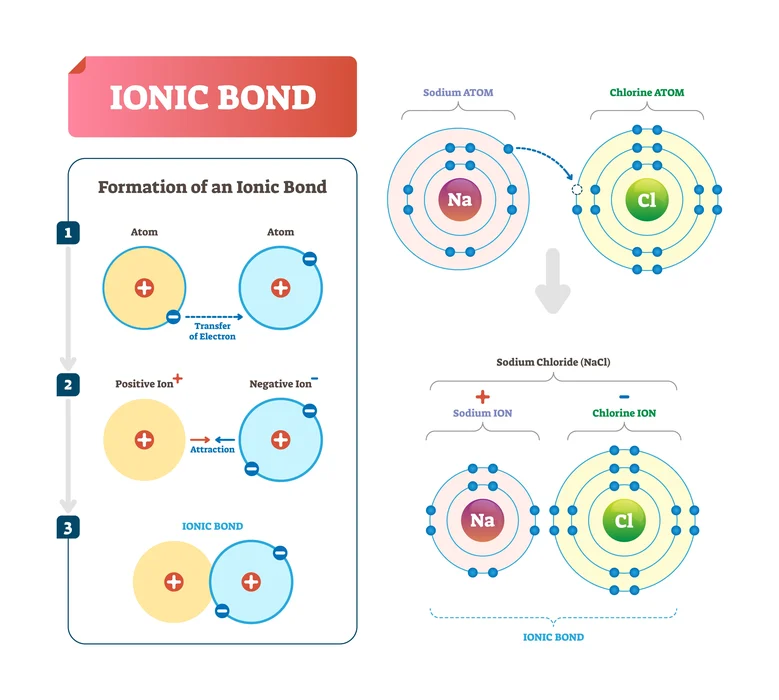
1.Ionic bond: When two atoms approach each other and have a large electronegativity difference, electrons, and anion forms are accepted. And the one that lost an electron will become an anion. An Ionic bond is formed as a result of the attraction caused by the positive and negative charge.
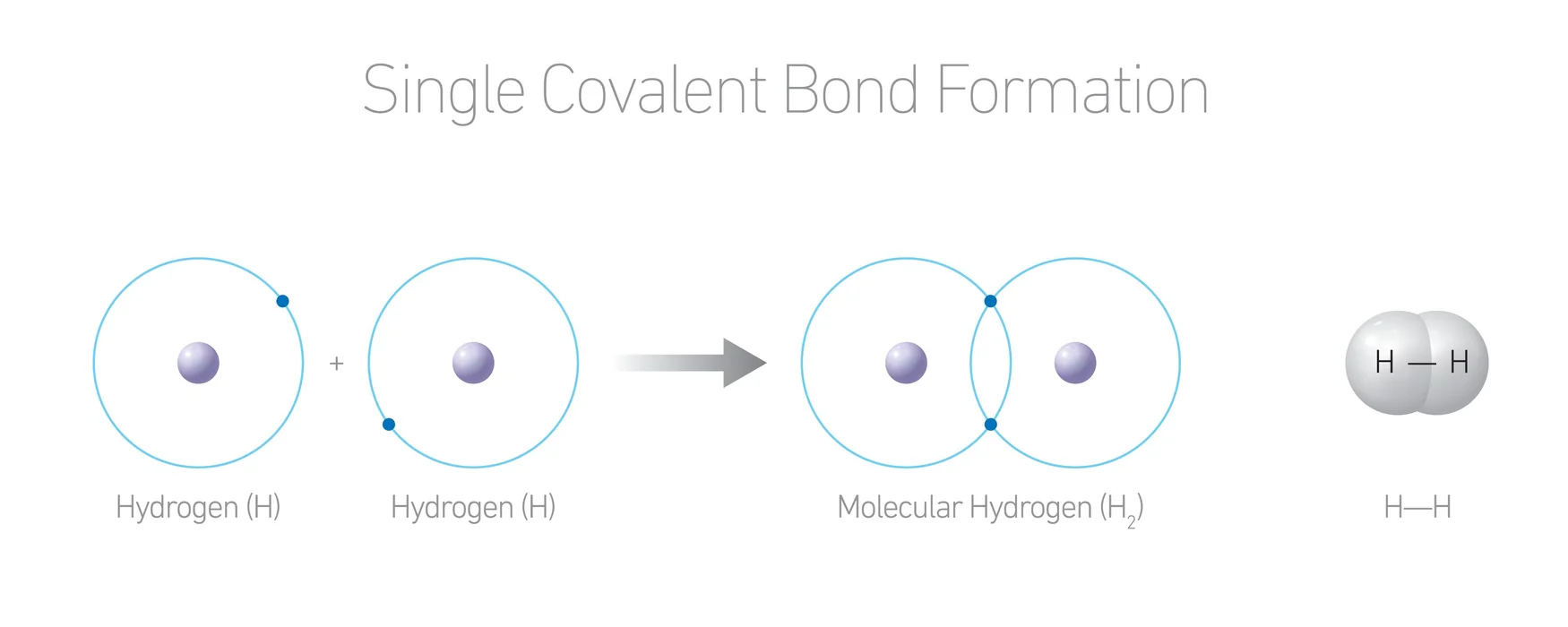
2.Covalent bond: When atoms with similar electronegativity differences approach each other, they share electrons. And this is a covalent bond.
Summary
Chemistry’s fundamental terms are atoms and molecules. Atoms are the fundamental building blocks of elements. A molecule is formed by the combination of different atoms using a chemical bond. These bonds could be covalent or ionic. Protons, neutrons, and electrons make up an atom. The size of an atom cannot be calculated precisely; only an approximation of size is possible.
Frequently Asked Questions
1. What exactly are isotopes?
Ans. Isotopes are atoms with the same atomic number but different mass numbers. The same atomic number denotes the same number of protons. And a different mass number means a different neutron number.
2. What is the mass number?
Ans. The mass number is the sum of protons and neutrons added to an atom of a chemical element. Lithium, for example, has a mass number of 7. Lithium has 3 protons and 4 neutrons.
3. What is the chemical formula?
Ans. A molecular formula is an expression used to represent a chemical compound that is the simplest whole-number ratio of the composition of elements present in a molecule.