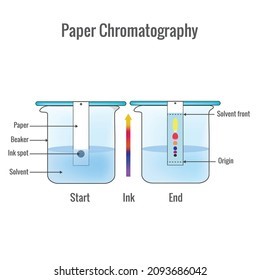
Introduction
In the lab, chromatography is used to dissect complex mixtures into their constituent parts. The process of separating the components of a mixture is known as chromatography. In order to accomplish this, a mobile phase and a stationary phase are utilised. Since various components in a mixture are subject to varying degrees of influence from the solvent, the mobile phase transports some of the mixture’s components while leaving the stationary phase behind As a result, certain parts will progress quickly while others lag behind. Each component has a unique Rf value. Considering how useful it is in both the purification and analysis processes, it can be found in a wide variety of contexts.
What is the Chromatography technique?
For analytical purposes, a mixture’s distribution into two phases provides a useful starting point for determining how to break it down into its constituent parts. Two such stages are the “mobile” and “stationary” states.
The non-moving phase is called the stationary phase, while the moving phase is called the mobile phase. A glass plate, column, sheet, or anything else is acceptable as the stationary phase. The mobile phase might be either a gas or a liquid.
Since it turns out that every type of molecule has its own unique characteristics. As a result, the Rf value will be unique to each. The Rf number describes how many solute molecules were transported for every one solvent molecule.
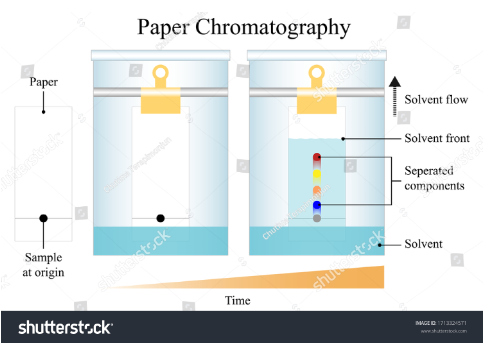
Chromatography
What is the Principle of Chromatography?
Chromatography operates on the idea of separating components based on their varied affinities to phases involved. Different components in a combination bind to the mobile phase and the stationary phase with varying strengths.
Applications of Chromatography in the Pharmaceutical Industry
In the pharmaceutical sector, chromatography is commonly employed as a quality control measure once a chemical has been manufactured.Many different types of chromatography have been used in the pharmaceutical sector. It is employed in both compound analysis and the industrial production of compounds. Separation of chiral substances has also been discovered to benefit from chromatography.
Applications of Chromatography in the Food Industry
Chromatography has been used for quality control in the food business. Chromatography is useful for determining what kinds of ingredients are in a given dish. Considering the importance of health, it is crucial to pack food with nutrients and vitamins. Several illnesses may be brought on by a deficiency in necessary vitamins and nutrients.
Applications of Chromatography in the Chemical Industry
In the chemical industry, it has applications in the chemical synthesis process. It acts as an assistant for obtaining a particular product. Chromatography has also been used in the pesticide industry for finding the presence of contaminants. For checking the pollutant present in water and air chromatography is used. So it is used in the chemical industry for monitoring several chemical reactions.
Applications of Chromatography in the Field of Molecular Biology
Biological separation using chromatography brings into the world considerable impact on the health and wellness of the people. Without this technique, the preparation method we employ, the synthesis of a particular molecule, etc. will be useless. It has helped researchers to find results faster and with purity. So it has a significant role in molecular biology too.
How is chromatography used in the environmental analysis?
Chromatography has wide application in the analysis of environmental issues. Understanding whether the air is polluted or not is very important since quality air is very essential for the proper living of species. Gas chromatography has been used for this case.
How is chromatography useful in Forensic Science?
Chromatography has a vital application in forensic science. For collecting information about the cause of death of a human, chromatography has been employed. It can be used for testing whether a person is infected with some poison, alcohol, drugs, etc.
Commonly employed chromatography techniques include:
Column chromatography: The stationary phase used in this chromatography is a column and a solvent is used as the mobile phase. Components are separated by their difference in affinity to the solvent. And is used to remove impurities present in a particular compound.
Ion-exchange chromatography: Separation based on the ions involved in a compound. An ion exchange resin is used as the stationary phase. For water purification, this technique is used.
Gel-permeation (molecular sieve) chromatography: Separation based on the size of the molecule is gel permeation chromatography. For finding the molecular weight of polymers. This technique is used.
Affinity chromatography: It is based on specific and highly selective reactions of components present in a mixture. Used for the purification of proteins and nucleic acids.
Paper chromatography: It involves the use of paper as a stationary phase and a solution or solvent as the mobile phase. It is used for purity checking in the pharmaceutical industry.
Thin-layer chromatography: Separation using a thin layer of adsorbent is thin layer chromatography. Mainly used to separate none volatile substances.
Gas chromatography (GS): Separating volatile chemical compounds is gas chromatography. And the mobile phase is a gas stream and a column is used as the stationary phase. It is used widely in the pharmaceutical and cosmeceutical industries.
Dye-ligand chromatography: Columns pasted with dye ligand act as a stationary phase and are separated with the use of affinity. It is used for protein purification.
Hydrophobic interaction chromatography: This separation of components is based on the hydrophobicity of components. Low water-soluble molecules are separated based on this technique. For example in protein purification.
Pseudo Affinity chromatography: The separation of protein molecules by the use of dyes that mimic ligands is pseudo affinity chromatography. It is used in protein purification.
High-pressure liquid chromatography (HPLC): It involves the use of liquid mobile phase under high pressure and thereby separating molecules from its mixture. It is used in the pharmaceutical industry for the identification of impurities present in it.
Summary
Chromatography is one of the analytical techniques that may be used to separate and purify molecules. It can be used in the pharmaceutical and food industries, among others. Although while analysis is where this technology shines most, it has also been put to use in molecule creation. Chromatography uses two phases—a mobile phase and a stationary phase—to isolate individual substances from a mixture. These phases also serve as a basis for categorising the various chromatographic methods. Methods including gas chromatography, gel permeation chromatography, paper chromatography, high-pressure liquid chromatography, and others are all included.
Frequently Asked Questions
1. What is the difference between flash and preparative chromatography??
Ans: Flash chromatography is used to quickly purify compounds, while preparative chromatography is used to purify compounds with higher resolution.
2. What is isocratic and gradient elution?
Ans: Isocratic elution is used to separate and analyze compounds with a constant mobile phase composition, while gradient elution is used to separate and analyze compounds with a changing mobile phase composition.
3. Which criteria must be followed before selecting a mobile phase?
Ans: Before selecting a mobile phase we need to consider whether it is soluble or insoluble in a particular component that is going to separate. And also need to check the polarity of the compound that has been employed.