Introduction
All science and technology are built on measurements. Every measurement is made by calculating an instrument which yields some ambiguity or doubt. This ambiguity is referred to as an error. This measurement flaw can be described in two ways:
1. Precision
Every measurement is dependent on the precision of the measuring tool and the skill of the person performing it. We won’t get the same result if we repeat a specific measurement because each result is susceptible to some experimental difficulty or inaccuracy.
2. Accuracy
When getting measurements, it is critical to believe these measurements. Both values indicate the degree to which a measurement is close to a known or acceptable value.
Define Accuracy
It is defined as the ability to relate a physical quantity’s true value to a measurement. When these difficulties or inaccuracies are reduced, the measurement becomes more precise.
Define Precision
Precision is defined by the smallest count of measurement equipment. Precision is greatest when the count is the smallest. Precision is the amount of information conveyed in terms of its digits; it indicates the proximity of two or more measurements to one another.
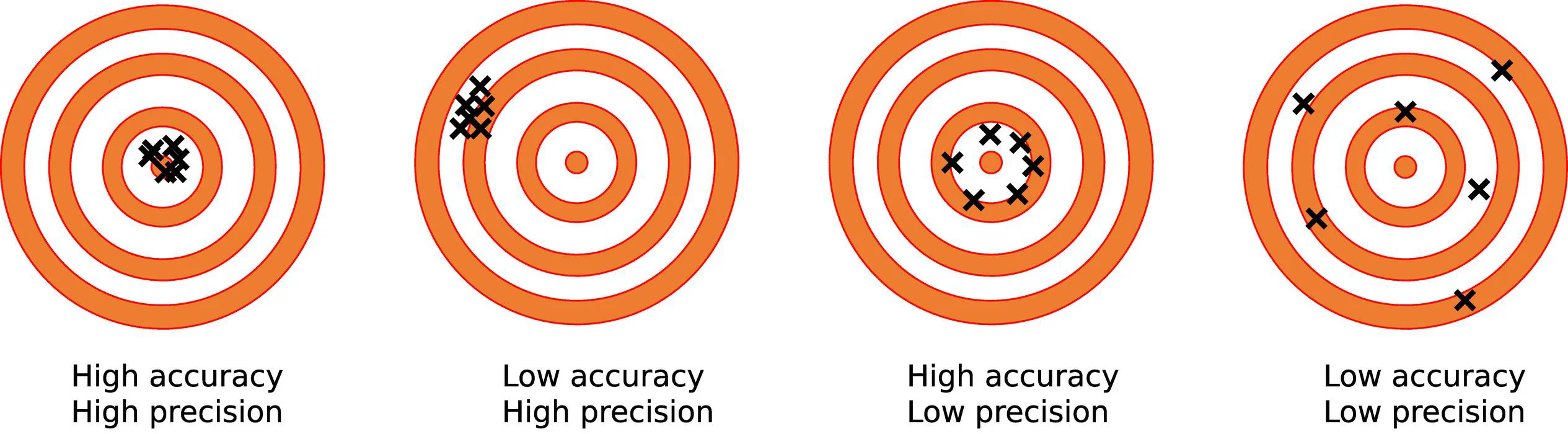
What are the differences between Accuracy and Precision?
| Accuracy | Precision |
| The near value of a measurement to the true value of a physical quantity is defined as accuracy. | Precision is defined as the slightest count of the measuring instrument. Or closeness to the actual readings of the same quantity. |
| Accuracy can only be dependent on a single factor or quantity. | Whereas, the precision can be altered or dependent on multiple factors. |
| Accuracy is expressed in the terms of the errors. | The precision is expressed in the terms of the deviation. |
| The determination of accuracy is dependent on a single measurement. | The determination of precision is dependent on multiple measurements. |
| Accuracy is dependent on precision. When results are accurate, they are also precise. | There is no dependence on accuracy. The results can be precise without being accurate. This shows no dependence of accuracy on precision. |
Summary
When experts consider error, they always think about accuracy and precision. It is defined as the ability to relate measurement to the true value of a physical quantity. Precision is defined as the measuring instrument’s smallest count. It is clear from the preceding explanation that the best scientific outcomes are only likely if they are both accurate and precise.
Frequently Asked Questions
1. According to one chocolate company, each bag of chocolate weighs 31.8 g. Jayant weighs two bags and discovers that they weigh 31.9 g and 32.3 g, respectively. How would Jayant describe the precision and accuracy of the first bag he measured?
Ans. The first bag’s claimed mass is correct. This is due to the fact that the brand specifies that each bag should contain 31.8 g, and the first bag did contain 31.8 gm. The claim for the first bag is not precise because the results are not identical.
2. How to determine Accuracy and Precision?
Ans. The accuracy of an experiment is calculated by the mean value of multiple measurements.
The precision of a set of measurements can be calculated by the standard deviation.
3. What is the relationship between accuracy, precision, and error?
Ans. The ability to relate the true value of a physical quantity to a measurement is defined as accuracy. When these difficulties or inaccuracies are reduced, the measurement becomes more precise. Precision is the ease with which a measurement can be replicated. Precision is defined by the measurement equipment’s smallest count. Precision is greatest when the count is the smallest. The precision of a set of values obtained by repeatedly measuring a quantity is defined as the closeness of the set of values obtained. As a result, more measurements will result in better precision, which will result in a smaller error, which will result in an improvement in accuracy.