Introduction
The discovery of calcium phosphate in bone tissue for the first time in 1769 marks the beginning of calcium phosphate’s usage in medicine. Calcium phosphates have played a key role in the field of bone tissue engineering. Calcium phosphate, the calcium salt of phosphoric acid, has several applications. Calcium phosphate inhibits the ability of the GI tract to absorb radium and strontium after oral consumption.
Phosphate is essential to the kidneys’ capacity to eliminate hydrogen ions, alters calcium concentrations, buffers acid-base equilibrium, and modifies calcium concentrations. Calcium phosphate’s phosphate ions likely react with hydrochloric acid in the stomach to neutralise the pH. Calcium phosphate is a source of calcium and phosphate ions that promote bone homeostasis and dental remineralization, respectively, in toothpaste and systemic circulation.
What is Calcium Phosphate?
Calcium ions \(\left( {C{a^{2 + }}} \right)\) and inorganic phosphate anions constitute the calcium phosphate category of chemicals and minerals. Some “calcium phosphates” contain oxide and hydroxide as well. Calcium phosphates, which are white solids of nutritional value, are present in a range of living organisms, including bone minerals and dental enamel.
\(C{a_3}{P_2}{O_8}\) is the chemical formula for calcium phosphate. It exists in milk as colloidal calcium phosphate, which consists of micelles bound to casein protein with magnesium, zinc, and citrate. Phosphoric acid and fertilisers are produced using several calcium phosphate minerals. Some calcium phosphates, when used in excess, can result in nutrient-rich surface runoff, which can cause eutrophication and algal blooms in receiving waters. It is soluble in hydrochloric acid and diluted nitric acid, but not in acetic acid or ethanol. Additionally, it is found in milk, bones, teeth, and coffee grounds, and it dissolves very slowly in water.
Characteristics of Calcium Phosphate
- Calcium phosphates are essential to geology, biology, medicine, dentistry, and industry.
- The solid rock known as apatite produces tribasic calcium phosphate, which is a complex and impure form of calcium phosphate.
- Calcium phosphate is a component of the mineral apatite, which is composed of phosphorite and other compounds.
- Its composition, solubility, stability, and structure influence its applications, formation, and processes of formation.
Calcium Phosphate Preparation
It can also be created by mixing solid calcium hydroxide with phosphoric acid. The following are examples of the chemical equation:
\[3Ca{{\left( OH \right)}_{2}}+2{{H}_{3}}P{{O}_{4}}\to C{{a}_{3}}{{\left( P{{O}_{4}} \right)}_{2}}+6{{H}_{2}}O\]
When calcium phosphate reacts with an aqueous solution of calcium hydroxide, dibasic calcium phosphate is produced. Contrarily, the excess phosphoric acid can be added to either a dibasic or a tribasic calcium phosphate solution and allowed to evaporate to produce monobasic calcium phosphate.
Structure of \(C{a_3}{\left( {P{O_4}} \right)_2}\)
Calcium phosphate is an ionic crystal made up of 3 calcium ions and 2 phosphate ions.
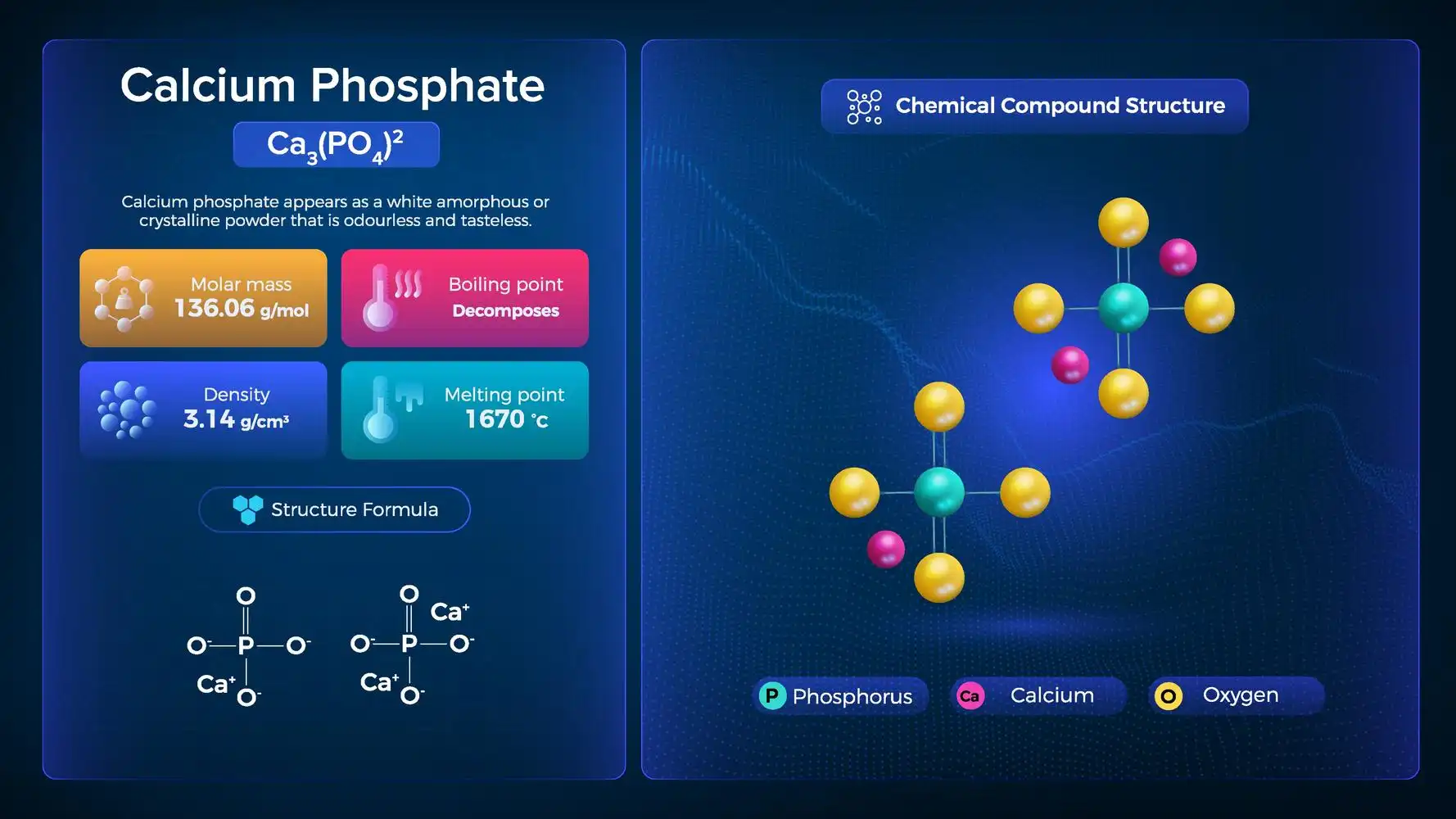
Properties of Calcium Phosphate- \(C{a_3}{\left( {P{O_4}} \right)_2}\)
The general properties of calcium phosphate are given below.
Solubility of Calcium Phosphate- \(C{a_3}{\left( {P{O_4}} \right)_2}\)
Calcium phosphate is insoluble in water but soluble in acids. The solubility of calcium phosphate has profound effects on the biological processes of resorption, the development of hard tissues, and pathological calcification.
Occurrence of Calcium Phosphate
Calcium phosphates can be found in nature in a variety of locations, and they are the primary minerals used to manufacture phosphate fertiliser and other phosphorus compounds.
Calcium and phosphorus supply the bulk of an animal’s mineral requirements. That’s why \(C{a_3}{\left( {P{O_4}} \right)_2}\) is such a popular and widely used supplement for animals. Furthermore, the rock phosphate dissolving tests prefer Dicalcium Phosphate Dihydrate because it is the most soluble of the hardly soluble calcium phosphate crystals.
Chemical fertilisers that dissolve in water, like diammonium phosphate or triple superphosphate, are the most common means of introducing phosphorus to soil. Given that phosphorus tends to dissolve in solutions at higher concentrations, processes involving precipitation are frequently favoured.
Health Hazards of Calcium Phosphate
When the amount of toxins ingested is greater than 2 gm/kg, the skin develops a sensitivity that is not seen in other people. If ingested, it could cause chemical pneumonitis. While calcium phosphate nanoparticles in and of themselves pose no danger to cells, their breakdown by lysosomes and subsequent uptake by endosomes can lead to an increase in intracellular calcium concentration. However, cells may eliminate calcium from the cytoplasm within a few hours unless exceptionally large quantities of calcium phosphate are utilised.
The cytotoxicity observed in some cell culture studies, in particular for the unfunctionalized particles, is likely due to the particles’ sedimentation and agglomeration on the cell layer, which results in a very high local particle concentration, subsequent cell death, and high absorption of particles. Calcium phosphate nanoparticles can enter the body through a number of routes, one of which is inhalation. No ill effects have been recorded except for those associated with chronic exposure to large particle doses.
Summary
Calcium phosphate can be found in crystalline or amorphous forms, and both have the same lack of flavour and aroma. However, it does not dissolve in acetic acid or ethanol. Dissolves very slowly in water. You can find it in foods like milk, meat, bones, and ground. Calcium phosphates have numerous uses across many disciplines, including biology, geology, industry, medicine, and dentistry. The composition, lability, stability, and structure of the material all play a role in its manufacture, uses, and applications.
Frequently Asked Questions
1. What are the side effects of taking too much calcium phosphate?
Ans: Symptoms of overdose of calcium phosphate include nausea/vomiting, loss of appetite, mental/mood changes, headache, weakness, tiredness.
2. Is calcium phosphate cement biodegradable?
Ans: Calcium phosphate cement, which comes in powder and liquid form, is a bioactive and biodegradable grafting material that, once mixed, sets as predominantly hydroxyapatite, though it may also contain unreacted particles and other phases.
3. Calcium Phosphate is acidic or basic in nature?
Ans. Calcium phosphate is basic salt, as it is a salt of weak acid (phosphoric acid) and slightly stronger base (calcium hydroxide).