Introduction
The measurement of a system or substance’s heat is called its temperature. The pace of an enzyme-catalyzed reaction typically increases as the temperature rises in most chemical processes. Temperature is the internal energy contained within a particular system. Temperature can be measured by a thermometer. Temperature is measured in degrees Celsius, which is written as °C, and can be also measured in Kelvin (K) and Fahrenheit (°F). Temperature alters the states of matter by reducing or increasing the interatomic distances.
Temperature Effect/Effect of Temperature
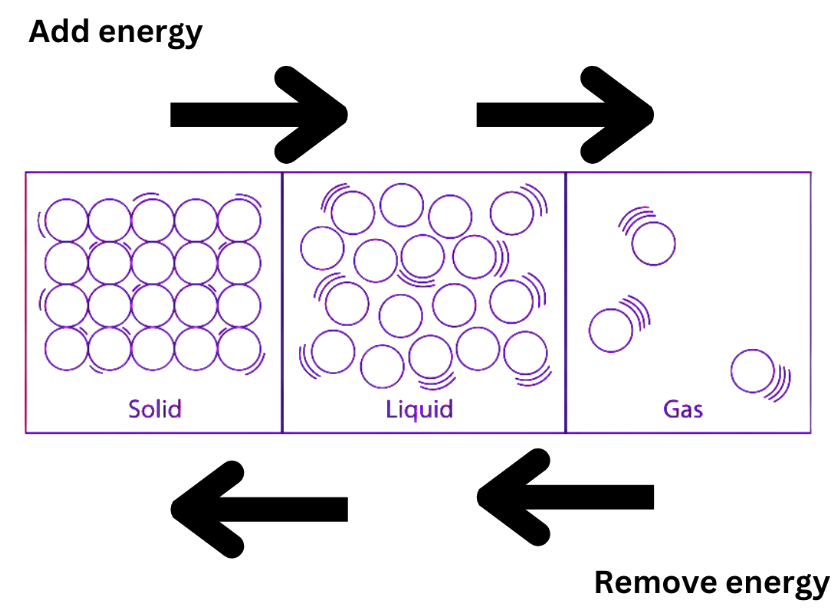
In several ways, the temperature has an impact on substances, processes, and enzymes. A substance’s state can be altered by varying its temperature. As the temperature rises, a solid can turn into a liquid, and as the temperature rises more, a liquid can turn into a gas. At high temperatures, a solid can be directly converted into a gas and this process is known as sublimation. Similar to the process where liquids may turn into solids at low temperatures, gases can become liquids by raising pressure while lowering the temperature. The rate of response of the transformation between the different states of matter is also positively or negatively impacted by temperature.
Learn More about Temperature and Heat. Check out more videos in 7th Class Science Lesson no 4.
The Effect of Change of Temperature on Solid State
As the temperature rises, the particles’ kinetic energy rises as well. As a result of the increase in kinetic energy, particles move faster and begin vibrating more quickly. The forces of attraction between the particles are weakened or eliminated by the heat energy. The particles start to travel more freely after leaving their fixed places. At some point, the solid melts and becomes a liquid. The melting point of a solid is the temperature at which it begins to dissolve into a liquid. When two distinct solid objects formed of the same substance are melted, they might combine to form a new one, a process known as fusion.
Effect of Change of Temperature on States of Matter
Heating and cooling are the two primary methods for converting states of matter. By applying heat, a solid can be transformed into a liquid. Similar to how a liquid may become a gas by heating. The opposite is also true; when a gas loses any of its thermal energy, it turns into a liquid. Further, removing a liquid causes its heat energy to solidify. The rising temperature causes a rise in the kinetic energy of the particles and the interspace between them. The force of attraction between particles is reduced by the increase in kinetic energy.
Effect of Temperature on Pressure
A physical force that is applied to an item by anything in touch with it is called pressure. The pressure can be estimated by the force per unit area. Three variables which affect how much pressure a gas exerts on the walls of the chamber of a container in which it is contained and surrounded by a vacuum. These factors are the quantity of gas within the chamber, the chamber’s size, and the gas’s temperature. The link between the pressure and temperature of gases can be explained, according to the gas law, which states that the pressure of a given volume of a specific gas is precisely proportional to its temperature at a constant volume. This relationship between pressure and temperature is what is meant by the term “pressure-temperature relationship.” It can be modelled as:
P∝T
A system’s temperature changes cause the molecules in the gas to move more quickly, increasing the pressure on the gas container’s wall. The system’s pressure rises as a result. The pressure likewise lowers when the system’s temperature rises. As a result, for constant volume, the pressure of a given gas is exactly proportional to the temperature.
The gas expands in volume at constant pressure as the temperature rises. The volume of the gas grows because it requires more space to move since the kinetic energy of the molecules increases as the temperature rises.
Effect of Temperature-Examples
The usage of light sticks or glow sticks is one illustration of how temperature affects the speed of chemical reactions. Chemiluminescence, a chemical process, occurs on the light stick. But neither is needed for nor produced by this reaction. The temperature has an impact on its pace. Precipitation reaction, activation energy, etc. are further examples.
Summary
The many states of matter are significantly impacted by temperature. The impact of temperature varies depending on the condition of matter. The kinetic energy of the state increases with temperature, yet the force of attraction varies depending on the state. The various states of matter are also impacted under constant pressure and volume. The pressure of the gas drops while the volume remains constant. The volume of the gas grows with constant pressure.
Frequently asked questions
1. What is the Liquid State of Matter?
Ans: Between the solid and gaseous forms of matter is the liquid state. Ice (solid), liquid as water, and gas as vapor are the three states of water. Although liquids do not have particular shapes, they do have specific volumes. The force of attraction in the liquid state is stronger than that in the gaseous state and weaker than that in the solid state. Atoms in this state have kinetic energies that are higher than those in solids, but lower than those in gases.
2. What Happens when the Temperature of a Gas Increases at Constant Pressure?
Ans. The temperature of a given system or gas container rises as the pressure in that system or container rises. While the pressure remains constant, the temperature rises, the velocity of the gas molecules increases, and the volume of the gas rise. As the gas’ temperature rises, the gas ascends into the atmosphere.
3. What is the Relationship between Pressure and Volume in Boyle’s Law?
Ans: When the temperature is maintained constant, Boyle’s Law states that the pressure exerted by a certain quantity of gas (the number of moles) is inversely proportional to the volume. The volume of the gas reduces with increasing pressure, and vice versa. The molecule tends to approach.