Introduction
CaO is the chemical formula for calcium oxide, which is a chemical compound. “Quicklime” is another name for this substance. In its cubic crystal lattice form, this chemical is extremely stable. As a result, its melting point is high, and it resists heat treatments quite well. Calcium carbonate ores are the primary raw material for this chemical. It can be found in powdered or crystalline form and is an amorphous material. The unadulterated form of this substance is a bluish-grey colour.
What is Calcium Oxide
- Calcium oxide (CaO) is an inorganic chemical that exists as a white crystalline powder.
- Quicklime is a type of lime that can also be used as a substitute for regular lime.
- It’s a lewis base and a metal oxide.
- Calcination of calcium carbonate ores, which eliminates carbon dioxide as a volatile contaminant and creates calcium oxide, is a common method of obtaining this material.
- This white, crystalline powder has the unique property of being able to undergo reversible reactions, making it quite desirable.
- It neutralises the acidity effects and dissolves easily in water. Its widespread industrial application can be attributed to the high temperatures produced when it reacts with water.
Structure of Calcium Oxide
The structure forms an ionic bond between one cation,\(C{a^{2 + }}\), and one anion,\({O^{2 – }}\). It is composed of six electrons in the outermost shell of an oxygen atom and two electrons in the outermost shell of a calcium atom.

Preparation of Calcium Oxide
- The mineral calcite \(CaC{O_3}\) found in limestone and seashells can be thermally decomposed in a lime kiln to produce calcium oxide.
\[CaC{{\bf{O}}_3}\left( s \right){\rm{ }} \to CaO(s) + C{O_2}\]
- Calcination refers to the process of making burnt lime. The process begins by heating the reactants to decompose them, but the temperature must be kept below their melting points or else the process will fail.
- At temperatures between 1070 and 1270 degrees Celsius, calcium carbonate is transformed by a process called calcination. Typically, a rotary kiln is used to host such processes. Limestone that has been burned and carbon dioxide gas are the reaction’s end products.
Calcium Oxide Properties
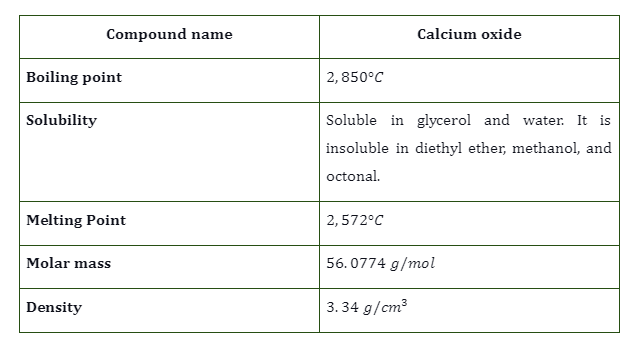
- Quicklime readily combines with water to form calcium hydroxide. It is an exothermic process. During hydration, it converts the powder form into a solid compound, calcium hydroxide as follows:
\(CaO\left( s \right){\rm{ }} + {H_2}O{\rm{ }} \Leftrightarrow \left( l \right)Ca{\left( {OH} \right)_2}\left( {aq} \right)\)
- Quick lime is a lewis base and neutralises the acidic oxides like \({\bf{A}}{{\bf{l}}_2}{{\bf{O}}_3},{\bf{Si}}{{\bf{O}}_2},{\bf{and}}\;{\bf{F}}{{\bf{e}}_2}{{\bf{O}}_3}.\) The reaction to these compounds produces molten slag which is basic. Therefore, quicklime is basic.
- As calcium oxide is a basic oxide, it combines with an acid to form salt and water. It is called a neutralisation reaction.
Calcium Oxide Uses
It is widely used in various industries as mentioned below:
- Compressed lime cartridges provide a very high exothermic reaction, which aids rock breaking in mining industry.
- CaO is used to separate sodium hydroxide from sodium carbonate during the papermaking process.
- CaO is used to detect the presence of water in a fuel storage tank.
- It’s the primary component in both cement and high-quality steel production.
- It is added to food to improve flavour.
- Caustic soda, flour treatment agents, and acidity regulators all rely on it as a crucial element.
- CaO is used to remove sulphur dioxide from water sources by flue-gas desulfurization, slurry, and solid sprays.
- To dehydrate and precipitate substances, this chemical is employed.
- When dealing with acidic soils, this is the approach to use.
What are the benefits Of calcium in the human body?
Calcium is the main source of Vitamin D. It helps in the formation of strong bones and is beneficial for teeth. It majorly helps in the proper functioning of the body like the heart, nerves, and muscles.
Interesting Facts about Calcium Oxide
- Calcium oxide gives a bright white light when heated to its melting point, though it has a very high melting point.
- It has a melting point of 2600°C and can handle high thermal temperatures.
- Lime or CaO is used to remove the acidic effect of acidic rainwater.
- Inhalation of calcium oxide irritates the eyes and skin.
- In the past, it was a myth that calcium oxide speeds up the decomposition of dead animals and humans.
Summary
CaO, or calcium oxide, has a very high melting point for a chemical substance. Extremely powerful and exothermic reactions occur when calcium oxide is mixed with water. In the process of doing so, calcium hydroxide is formed, which is both corrosive and thermally active (reaching temperatures of 800 °C). Although calcium oxide does not present a fire hazard on its own, when combined with water it generates enough heat to ignite flammable materials.
Frequently Asked Questions
1. How does calcium oxide react with hydrochloric acid?
Ans. It forms a salt calcium chloride along with water when calcium oxide reacts with hydrochloric acid.
\[{\bf{CaO}}{\rm{ }} + {\bf{2HCl}} \Leftrightarrow {\bf{CaC}}{{\bf{l}}_2} + {{\bf{H}}_2}{\bf{O}}\]
2. How is calcium oxide used in water treatment?
Ans: Calcium oxide is used in water treatment to remove impurities such as carbon dioxide, sulphur dioxide, and hydrogen sulphide.
3. Why is calcium oxide more hazardous than calcium hydroxide?
Ans. Calcium oxide is a substance that readily reacts with water to generate calcium hydroxide, whether that water is in the air, on your skin, or somewhere else. When calcium oxide reacts with water, it releases a great deal of heat, making it not only corrosive but also potentially dangerous because of the risk of burns.

