The molar mass of a molecule is its weight. Expressed in Daltons. When dealing with the mass of a single or specific well-defined molecule, molecular weight is more commonly used than molecular weight when dealing with sample-weighted averages.
Molecular weights of small to medium-sized molecules are measured by mass spectrometry and used to determine the elemental composition of molecules or compounds.
What is Molecular Mass?
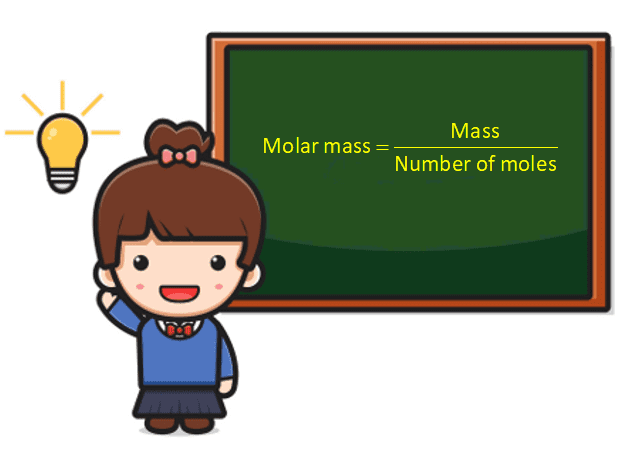
Molecular mass is also called molecular weight. The molecular weight of a molecule is its weight or mass. Different molecules of the same compound can have different molecular weights because elements have different types of isotopes present in compounds. The ratio of molecular mass to combined atomic mass is a measure of relative molecular weight. Molecular weight and molecular weight are separate but related concepts. The molar mass of a substance is defined as the mass divided by the number of moles of that substance. The unit of molar mass is g/mol.
How to Find Molecular Mass?
To calculate molecular weight, first, use the periodic table to determine the atomic weight of each element. Multiply the number of atoms by the atomic mass of each element to sum the masses of all elements in the molecule.
The Formula of molecular mass/ molecular mass equation
The formula of molecular mass or molar mass is.
How to Calculate Molecular Mass?
A compound’s total mass is referred to as its molecular mass or molecular weight. It is equal to the sum of the atomic masses of all elements.
Molecular Mass Examples
There are some examples of molecular mass:
H2O: In the periodic table, hydrogen has an atomic mass of 1u, and oxygen has an atomic mass of 16u. As a result, the molecular mass of a water molecule can be calculated as follows:
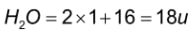
Hence, the molecular mass of water molecules is 18u.
NH3: In the periodic table, the atomic mass of hydrogen is 1u and the atomic mass of nitrogen is 14u. As a result, the molecular mass of an ammonia molecule can be calculated as follows:
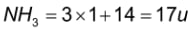
Hence, the molecular mass of ammonia gas is 17u.
CaCO3: In the periodic table, calcium has an atomic mass of 40u, carbon has an atomic mass of 12u, and oxygen has an atomic mass of 16u. As a result, the molecular mass of calcium carbonate can be calculated as follows:
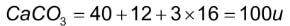
Hence, the molecular mass of calcium carbonate is 100u.
CaCl2: In the periodic table, calcium has an atomic mass of 40u, and chlorine has an atomic mass of 35.45u. As a result, the molecular mass of calcium chloride can be calculated as follows:
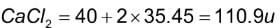
Hence, the molecular mass of calcium chloride is 110.9u.
The Molecular Mass of Compounds
The molecular mass of a compound can be found by using the following steps.
- Determine the compound’s molecular formula.
- Determine the atomic mass of each element in the compound using the periodic table.
- Multiply the atomic mass of each element by the number of atoms in the compound. In the molecular formula, this number is denoted by the subscript next to the element symbol.
- Add these values for each atom separately.
- The total value will be the compound’s molecular mass.
The Molecular Mass of Elements
An element’s molecular mass is the sum of the atomic masses of its constituent atoms. Using the periodic table, calculate the atomic mass of each element.
Conclusion
Molecular mass is defined as the sum of the atomic masses of the elements present in a molecule, whereas molar mass is the ratio of compound mass to a compound number of molecules. Mechanical properties generally increase as molecular weight increases. A polymer’s molecular weight is directly related to its properties (strength, processability, and brittleness). The smallest molecular mass is hydrogen. Molecular mass is useful for analyzing experiment results. Knowing the molecular formula allows you to calculate the molecular mass.
Also Read: Formula Unit Mass and How is it Calculated?
Frequently Asked Questions
1.What is the Molecular Mass Equivalent to?
Ans: Molecular mass is a number equivalent to a molecule’s amount of nuclear masses.
2.What are the Characteristics of Polymers?
Ans: A polymer is a large molecule composed of chains or rings of linked repeating subunits known as monomers. Polymers have high molecular masses because they are made up of many monomers, and they also have high melting and boiling points.
3.What is a Compound Composed of Identical Molecules?
Ans: A compound is a substance made up of identical molecules made up of atoms of two or more chemical elements. Atoms of over 100 different chemical elements make up all matter in the universe, both in pure form and in chemical compounds.