Introduction
In 1913, Neil Bohr introduced the Atomic Model, which was based on Planck’s quantum theory of radiation. In overcoming the limitations of Rutherford’s atomic model and describing the hydrogen spectral lines. One such model appeared to be very effective in describing the atom’s stability, as well as the line spectra of an H atom. This model hypothesis correctly predicted smaller atoms such as hydrogen, but when larger atoms were observed, poor phantom assertions were made. However, it does not describe the Zeeman effect, atomic spectra, the Stark effect, or Heisenberg’s Uncertainty Principle.
An Overview of the Bohr Atomic Model
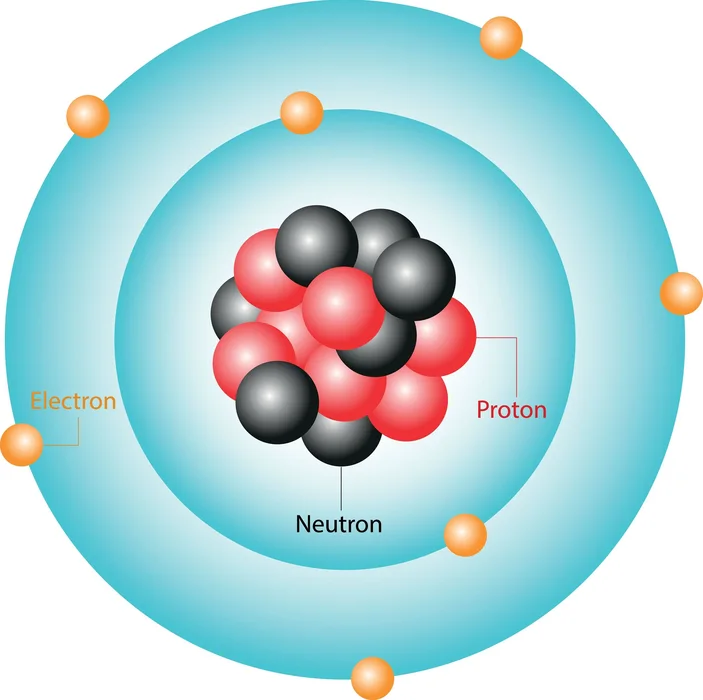
According to Bohr’s Atomic Model, an atom is made up of a tiny, positively charged nucleus surrounded by electrons that move in circular orbits all over the nucleus, attracted by electrostatic forces. He was also awarded the Nobel Prize in Physics for his contributions to atomic structure.
The Bohr Atomic Model’s Postulates
1. Orbits are allowable circular trajectories in that electrons travel all over the nucleus.
2. Because each orbit was associated with a specific amount of energy, they were referred to as energy levels and energy states.
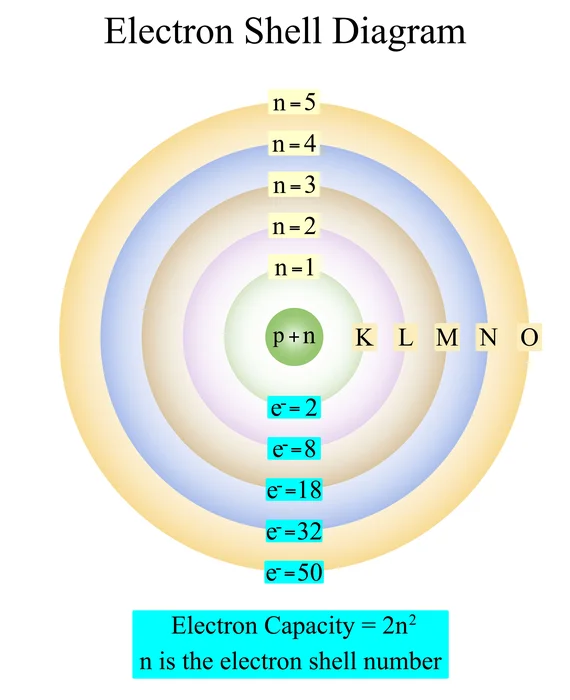
3. Shells of energy have been labelled as 1, 2, 3, 4,… or even K, L, M, N…., and so on. The energy level closest to the nucleus is denoted 1 and is known as the K shell.
4. When travelling at a certain energy level, an electron does not lose or gain energy. In such a given energy state, an electron’s energy appears to be fixed as well as stationary. This is referred to as the normal or ground state.
5. When an electron moves from one orbit to the next, it both emits and absorbs energy. When it travels from a higher energy state to a lower energy state, it releases energy, and when it travels from a lower energy state, it consumes energy.
6. Planck’s equation calculates the absorbed and released energy as the difference between the energies of the energy states.
Bohr’s Atomic Model’s Limitations
1. Bohr’s concept did not explain the atomic spectrum of elements with more than one electron.
2. This does not account for the Zeeman effect, which occurs when a magnetic field breaks spectral lines into densely closed lines.
3. It also fails to illustrate the Stark effect, which occurs whenever spectral lines are broken into fine lines by an electric field.
4. According to Bohr, electrons’ circular orbits appear to be flat. However, a new study shows that an electron travels in 3 dimensions across the nucleus. Because light electrons have a dual nature, this would be centred on de Broglie’s idea.
5. Bohr’s atomic model would not adhere to Heisenberg’s uncertainty principle. According to this theory, determining the precise position and momentum of a tiny circulating particle like an electron with extreme certainty appears to be difficult. As a result, electrons follow a well-defined circular path.
6. Bohr’s hypothesis can never explain the shapes or the structure of molecules. This incorrectly considered large-sized atoms while providing sufficient information about smaller atoms.
Summary
Niels Bohr’s atomic theory includes definite-size electrons as well as energies travelling in orbits around a central nucleus, similar to how planets orbit the sun. To summarise Bohr’s atomic model, the energy states of electrons are focused on the size of such orbits. As a result, electrons in tighter orbits would have less energy. Atoms are unstable because electrons move to drive down orbits, resulting in radiation. Because the electron appears to have no lower orbit to which it can jump, an atom within the smallest orbit has now become completely stable. As a result, it was hypothesised that such an electron could move between these orbits by absorbing and losing photons.
Frequently Asked Questions
1. What exactly is the Bohr Atomic Model Theory?
Ans. Bohr was the first to discover not only that electrons revolve around the nucleus in distinct orbits, but also that the total number of electrons in an element’s outermost shell can be used to define its properties.
2. What is preventing atoms from collapsing?
Ans. The electrons of an atom are kept from collapsing within the nucleus by balancing kinetic and potential energy.
3. What is the radius of a Bohr orbit?
Ans. The Bohr radius, denoted by ‘r,’ has been defined as the mean radius of an electron’s orbit around the nucleus of an H atom in its initial state. Its radius has become a standard value, roughly equivalent to \({5.2917710^{ – 11}}m.\).