Introduction
The shortest form of polymers is called monomers. Depending on their characteristics, preparation process, and many other factors, they are classified into two types- Natural and Synthetic polymers. The polymers which are generated naturally are called natural polymers, whereas polymers made by humans in some synthetic processes are called Synthetic polymers. There are different kinds of useful synthetic polymers. Thermosetting polymers are one kind of them.
Define Thermosetting Polymer
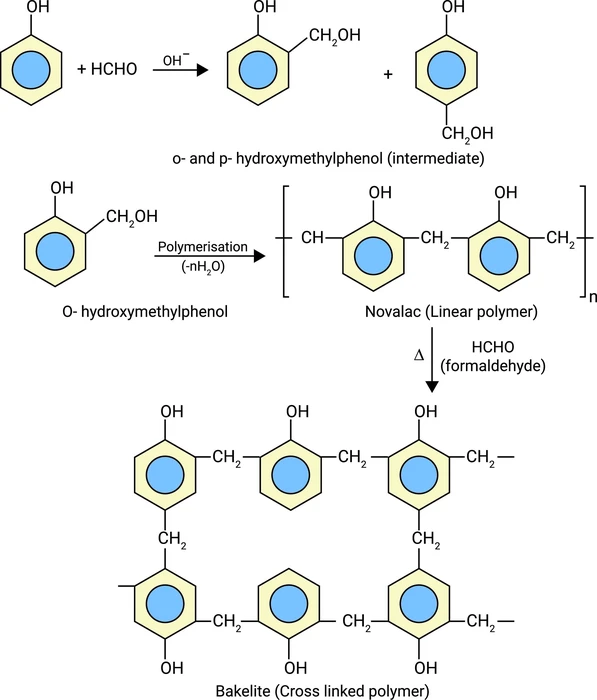
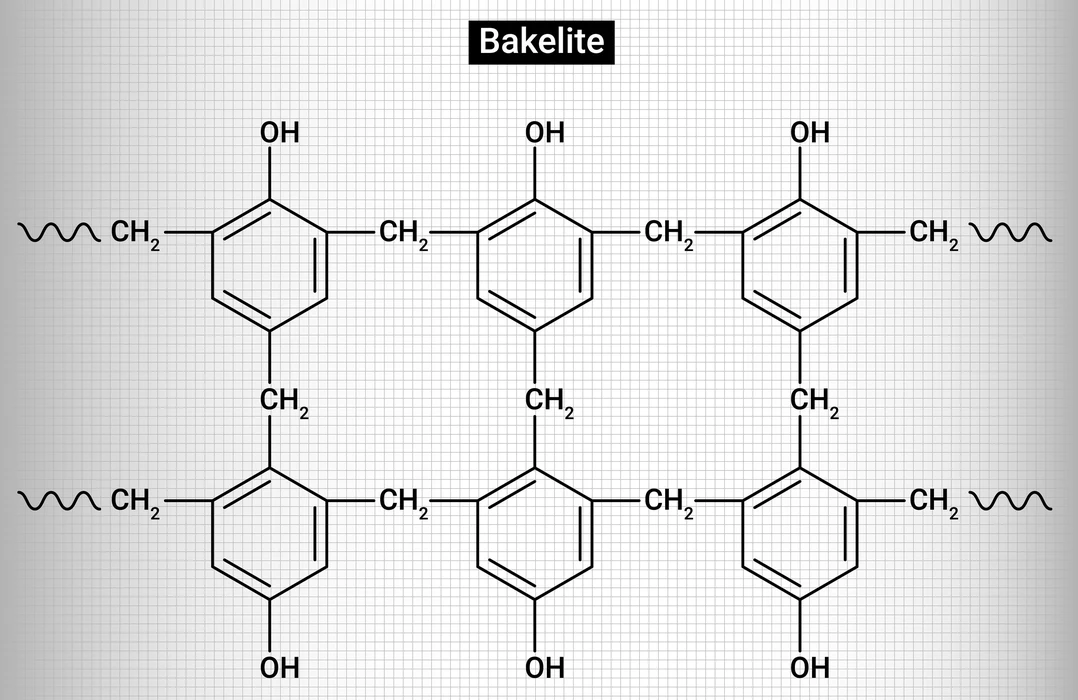
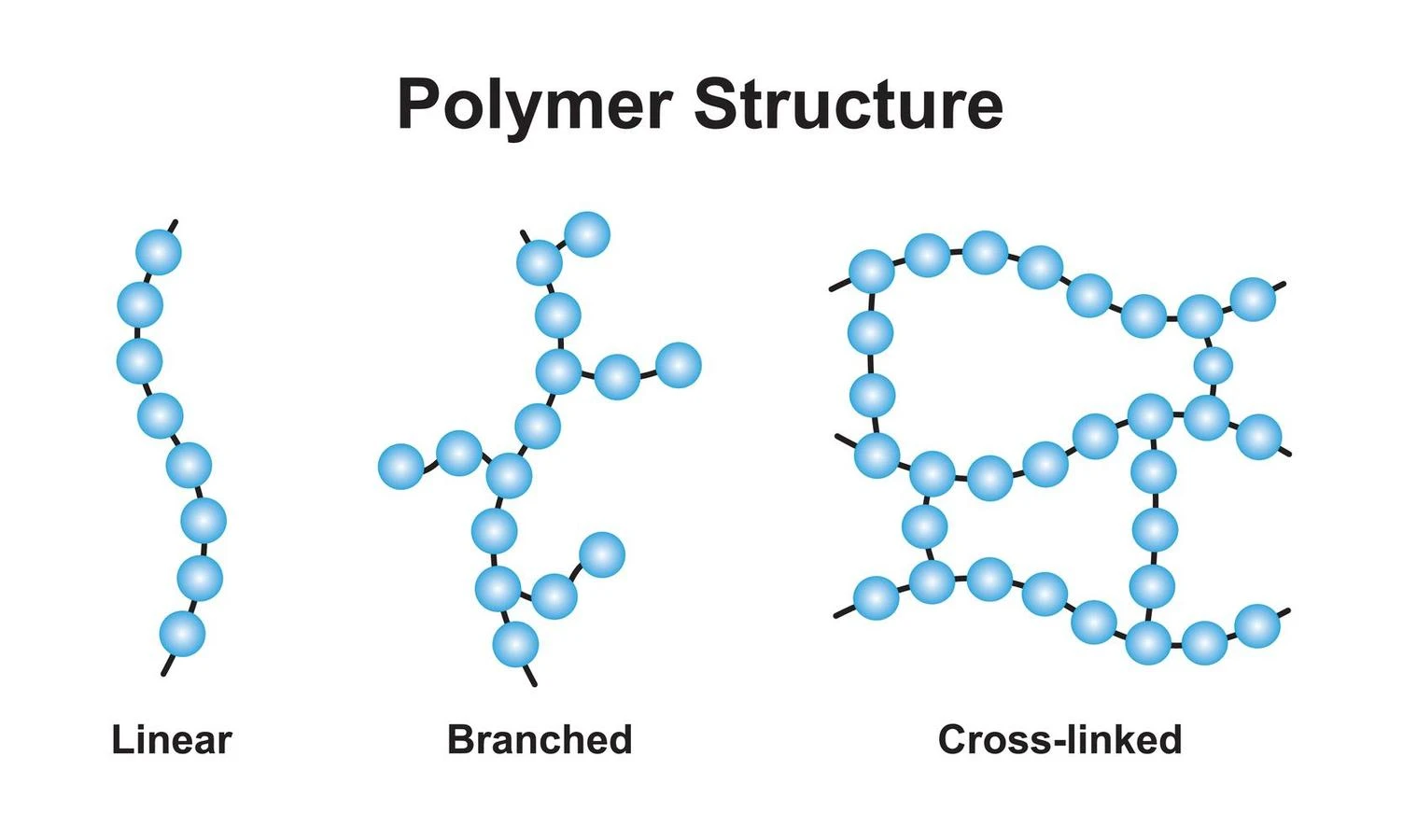
A thermosetting polymer can be called by another name ‘Thermoset’. By heating the thermosetting polymer, it undergoes irreversible changes. It has a cross-linking structure, forming a three-dimensional network. Initially, these polymers remain in a liquid state and they are soft. But after heating, they become harder. The rate of cross-linking in them increases with an increase in temperature, pressure, and amount of catalysts. Bakelite, Duroplast, and Melamine resin are examples of thermosetting polymers.
Features of thermosetting polymers
This type of polymer has unique properties unlike the other types of polymers.
- Monomers are condensed easily and form polymers. Thermosetting polymers are types of condensation polymers.
- Due to the high cross-linking process, the molecular weights of these are very high.
- Thermosetting polymers possess exceptionally high melting points and very strong tensile strength.
- It is not reprocessed to its original state because of the intense cross-linking. It is created permanently. In other words, the reaction mechanism is irreversible.
Synthetic pathway of Thermosetting Polymer
The initial stage of thermosets is a resoluble, molten, and insoluble state. Next, it transforms into a thermoplastic-like substance that is slightly soluble and has certain reversible properties. Following this phase, polymers undergo cross-linking and hardening. Finally, a 3D network containing polymers is formed. There is also another method of preparation containing three steps.
- Moulding by compression: Here a heated mould cavity is used and then it is compressed to produce the plastic. With an increase in the application of heat on mould, the chemical interaction also increases.
- Transfer moulding: The utilization of a transfer pot is common in this kind of manufacturing, and heating the mould within it, will enhance the materials’ flow.
- Injecting the mould: Screws are employed in the injection moulding process to inject the high volume of polymer into the appropriate moulds. Additionally, it is discovered that the liquification of these polymers decreases the polymers’ viscosity.
Advantages of Thermosetting Polymers
- For thermosets, both chemical and heat resistance are excellent. It’s employed for packed items and has a strong deformation resistance.
- Thermosets are beneficial for wet paste formulations since they contain minimal solvent.
- The linked chains cannot freely travel because the use of heat turns them hard.
- Epoxy and phenolic resins have a wide range of uses, particularly in the production of circuit boards and containers.
Disadvantages
- It cannot be converted to its original state after it has formed.
- With thermosets, fine surface quality is not achievable.
- This polymer should be managed with utmost care.
Summary
Bulk molecules, known as polymers, have numerous industrial uses. There are two categories of polymers, both natural and artificial. Artificial synthetic polymers are generated by executing specific chemical changes. Because they’re irreversible polymers, even after heating, they cannot be reverted to their initial position. Therefore, it is an extremely rigid and hard polymer. During the curation process of thermosets, chemical interaction occurs in them. So remoulding is not possible. But for thermoplastic polymers, no chemical interaction occurs during curation. Due to the possibility of remoulding in thermoplastic polymers, thermosets are a better option than thermoplastic polymers.
Frequently Asked Questions
1. What are the reasons that thermosets don’t melt?
Ans: During the curation process, cross-linking occurs in the polymers to produce an unbreakable and permanent bonding. It is clear that even with the application of strong heat, thermosets can’t be melted.
2. What are the reasons behind the ignition of thermosets?
Ans: Thermosets possess significant melting points, but as soon as they reach a particular temperature and harden, their parts and physical features become fixed. They cannot be transformed back into their original shapes or sold for scrap again. Rather the substance will just burn or char.
3. Do thermosets participate in the crystallization process?
Ans: It is found that thermosets are amorphous in nature. Due to the high cross-linking process in their structures, they don’t crystallize.