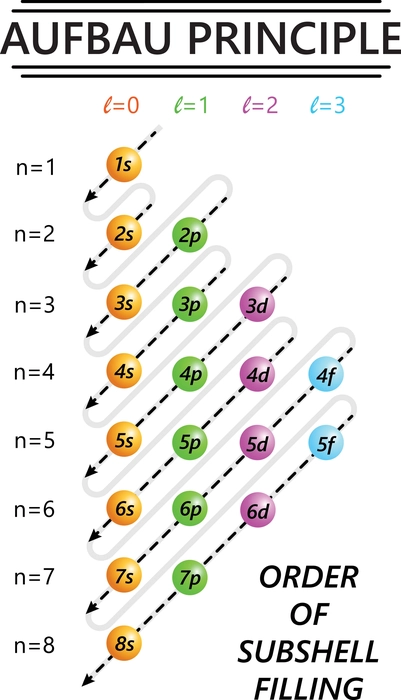
Introduction
Atomic orbitals are the three-dimensional spaces that surround the nucleus and are most likely to contain an electron. The atomic orbitals have been combined to create molecular orbitals. They obtain orbitals in quantum theory, some of which are electron shells in the s, p, d, and f configurations. Although orbitals come in a variety of shapes and sizes, their square can be used to estimate their size or even shape. A total of two, six, ten, or fourteen electrons could fit in the s, p, d, and f subshells, respectively. The particular arrangement of electrons within orbitals in such an atom determines the majority of the chemical compositions of that atom. Depending upon the energy over its electrons, every orbital class seems to have a distinct form. The s orbital has a spherical geometry. The p orbital has the form of a dumbbell, as well as 3p orbitals, which vary in their arrangement across a 3-dimensional axis.
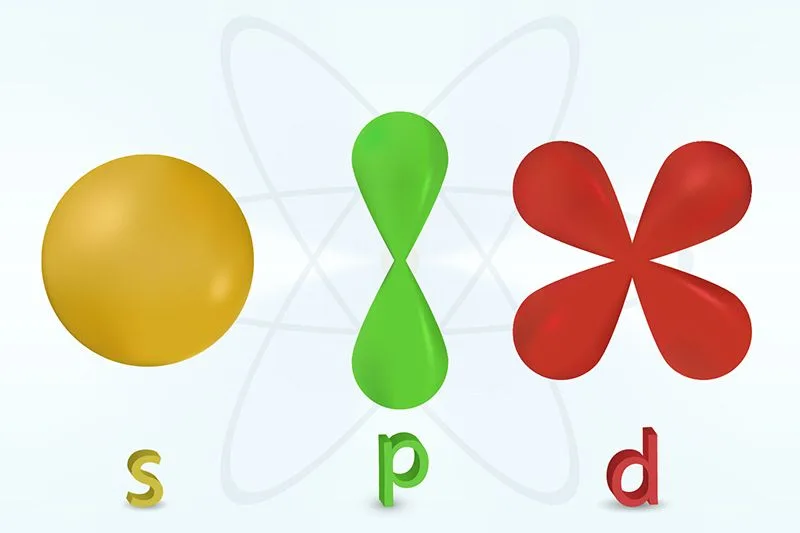
Define Atomic Orbitals
Atomic orbitals are numerical values that provide additional details about the waveform of electrons that inevitably surround atom centers. In the fields of quantum systems and atomic theory, specific mathematical expressions are frequently employed to determine the likelihood of detecting an electron at even a specific area surrounding the nucleus of an atom. The term “atomic orbital” may also refer to the region above an atom’s nucleus where there is the greatest chance that a particular electron will become accessible. Several quantum numbers affect each atomic orbital’s characteristics:
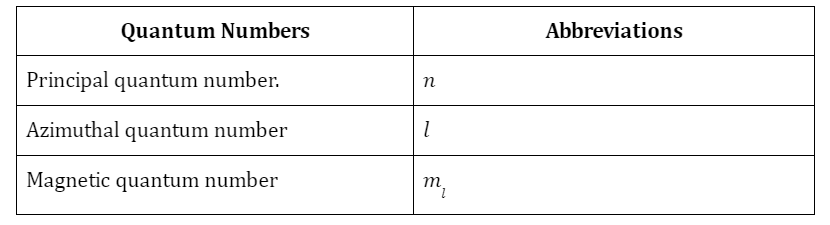
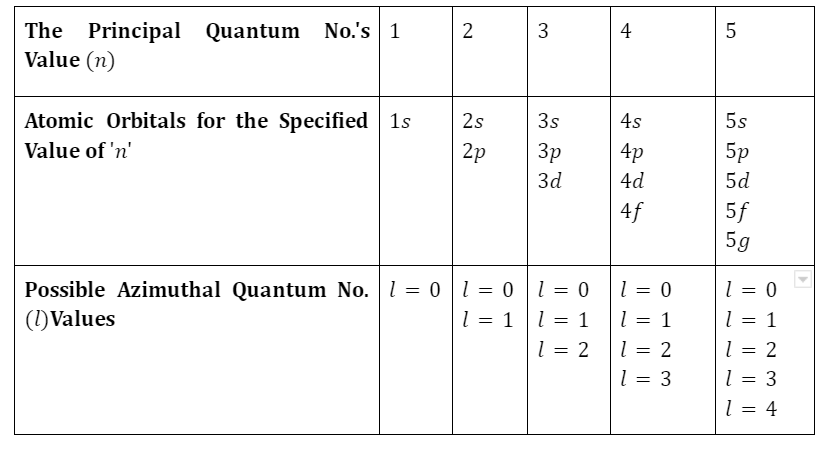
Table of All Possible Atomic Orbitals, where the Value of ‘n’ Ranges from 0 to 5
What do you mean by Atomic Orbital Theory?
An atomic orbital is a statistical term that describes the location or even waveform behavior of such an electron in an atom in both atomic theory and quantum field theory. These electrons, each of which has a distinct spin quantum number s, can fit into any one of these orbitals up to a maximum of two. One such formula can be used to determine whether it is possible to find an electron inside the nucleus of any atom at any particular location.
Summary
Atomic orbitals seem to be the regions around the nucleus of an atom where electrons have often been observed at that particular time. This is a mathematical concept that characterizes the wave-like activity with 1-2 electrons in an atom. Electrons inhabit low-energy orbitals (near such nuclei) before electrons approach higher-energy orbitals. When there is an option of equal-energy orbitals, then occupy the orbitals freely as feasible. That filling of orbitals on its own is termed Hund’s law when applicable. Atomic orbitals are typically denoted by a series of digits as well as letters representing unique features of such electrons linked only with orbitals, including 1s, 2p, 3d, as well as 4f. Primary quantum numbers are values that further indicate levels of energy.
Frequently Asked Questions (FAQs)
1. Is it possible to have an orbital without an electron?
Ans. An orbital’s characteristics are more like the electron residing inside it. This is standard procedure, however irrational this could appear, to refer to ‘Empty orbitals.’ The characteristics of unoccupied orbitals are the same as those computed for electrons within them.
2. What is perhaps the greatest number of orbitals possible?
Ans. The values n=3 & l=1 indicate that it has been a 3p-orbital, however, the number \(f(m_{l}=0)\) indicates that it is indeed a \(3p_{z}\) in origin. As a result, the specified quantum number can only identify one orbital, namely 3p_z.
3. How do electrons fill orbitals?
Ans. According to the Aufbau principle, electrons first occupy lower-energy atomic orbitals before moving onto the higher ones. Based on this method, we may forecast the electronic structure of atoms and ions.