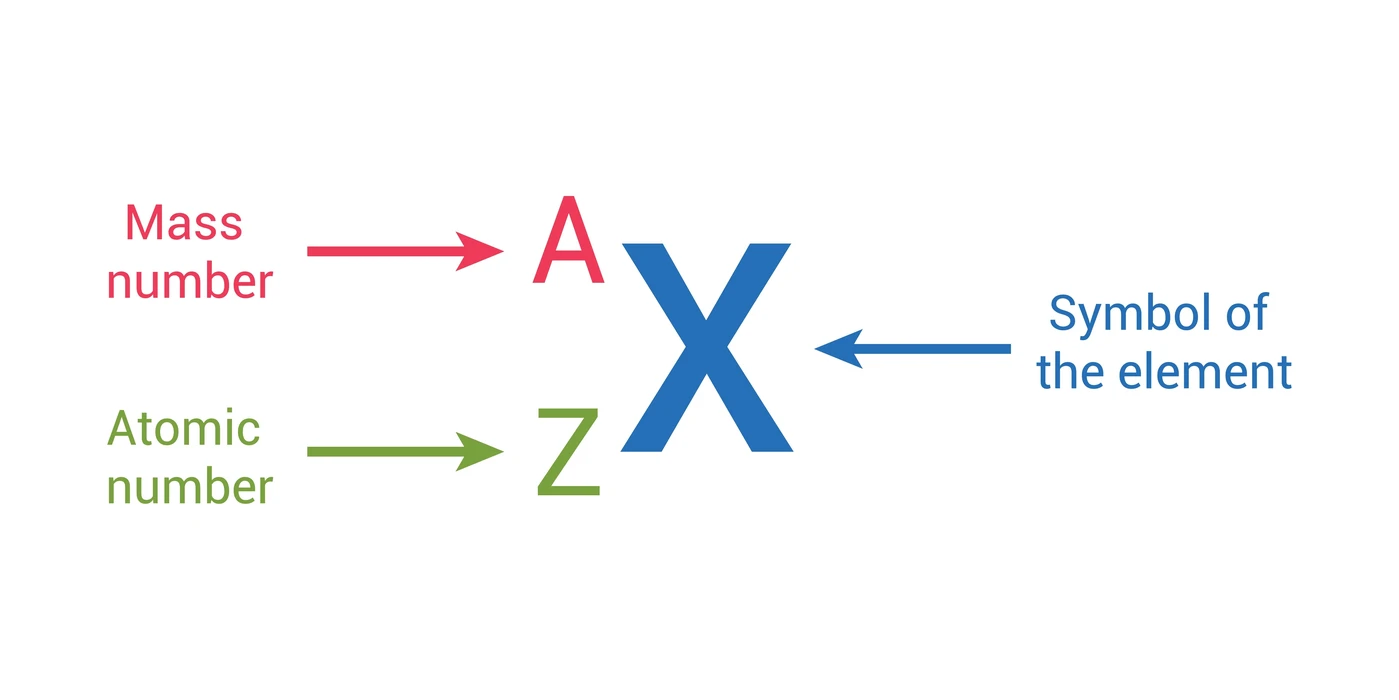Introduction
The symbol for an element is a single letter or two-letter abbreviation of the element’s full name. These symbols are helpful when writing out lengthy chemical equations that need the usage of shortened forms of complexes and elements. The number of electrons (\({e^ – }\)) and protons (\({p^ – }\)) in an atom can be determined from its atomic number (Z). In chemistry, an element is defined as a pure substance made up entirely of atoms having the same number of protons in their atomic nuclei.
What are the First 20 Elements of the Periodic Table?
here is the list of 20 elements with symbols and Atomic Number
| Atomic number | Symbol | Element |
|---|---|---|
| 20 | Ca | Calcium |
| 19 | K | Potassium |
| 18 | Ar | Argon |
| 17 | Cl | Chlorine |
| 16 | S | Sulfur |
| 15 | P | Phosphorus |
| 14 | Si | Silicon |
| 13 | Al | Aluminum |
| 12 | Mg | Magnesium |
| 11 | Na | Sodium |
| 10 | Ne | Neon |
| 9 | F | Fluorine |
| 8 | O | Oxygen |
| 7 | N | Nitrogen |
| 6 | C | Carbon |
| 5 | B | Boron |
| 4 | Be | Beryllium |
| 3 | Li | Lithium |
| 2 | He | Helium |
| 1 | H | Hydrogen |
Master the Periodic Table! Learn about all 118 elements, their symbols, and atomic numbers in our easy-to-understand article. Click here to start exploring now!
Importance of the Atomic Number of An Element in a Periodic Table
The number of protons in the nucleus or the number of electrons (\({e^ – }\)) in a neutral atom is equal to the atomic number. It determines the element’s position in the periodic table.
Eight is the atomic number of oxygen. This means that in the ground state of the nucleus, there are eight protons. There are eight electrons (\({e^ – }\)) in all. NaCl, with an atomic number of 11, also possesses 11 protons in its nucleus. There are 11 electrons in orbit around the nucleus.
Since the number of electrons in an atom is also its atomic number, the electrical configuration of an atom may be easily predicted using just the atomic number. Because the atomic numbers in the contemporary periodic table increase, this is a very important fact. One final factor that is crucial in determining the properties of an element is its atomic number. The nature of chemical bonds is determined by the number of valence electrons (\({e^ – }\)).
The periodic table’s atomic number is significant due to following reasons:
- If we order elements according to their atomic mass, we also need to list their isotopes, which makes the periodic table too long. However, if we arrange the periodic table according to the atomic number, we don’t need to write the isotopes.
- If the periodic table were ordered by atomic mass, the element hydrogen (H), which has three isotopes, would occupy three separate spots.
- The electrical configuration or valence electrons of an atom can be determined by its location in the periodic table, which is in turn determined by its atomic number.
- The organisation of the elements according to their atomic numbers is simple to learn and copy.
- If we know the atomic size based on the atomic number, we can use that information to make educated guesses about other properties of the element, such as its ionisation energy, electron affinity, and electronegativity.
- Atomic numbers allow us to determine an element’s oxidation state and valency by referencing its electronic configuration.
- Metals (+ve charge elements) can be distinguished from nonmetals via their oxidation states (-ve charge elements).
How are the First 20 Elements of the Periodic Table Useful for us
The first 20 elements of the periodic table are important to us because they are the basis of all known forms of life. We can’t survive without these things in our bodies. Proteins, nucleic acid, and other building blocks are made from them. The first 20 elements of the periodic table are the most abundant elements on the planet. Some of the most important elements are:
- Oxygen– It is required for respiration. The energy-producing mechanism that governs the metabolisms of most life forms is respiration. Humans, like many living beings, need oxygen to breathe. It is produced during photosynthesis in plants as well as various microorganisms.
- Carbon– It accounts for 18% of the body. It can be found in protein, sugar, & and other vital substances such as glucose. It can also be found in fossil fuels such as petroleum, CNG, & others.
- Aluminium– Because it is malleable as well as soft, aluminium is utilised to select items such as utensils, aeroplane parts, window frames, & so on.
- Silicon is a semiconductor that is utilised in computer chips.
- Phosphorus– In the armed services, phosphorus is utilised to build weapons. It is a crucial component of ATP, the body’s energy currency.
- Calcium -It aids in bone strength maintenance.
Noble Gases in the First 20 Elements of the Periodic Table
The noble gases are a set of six non-reactive gases found at the far right of the periodic table. They are located in the final group of the periodic table, Group 18. They lack aroma, colour, combustibility, and reactivity and have no discernible flavour. These subatomic particles can glow and carry an electric current. One organic chemist called these elements “lazy” because they don’t react with anything. Some examples of noble gases are radon, helium, xenon, krypton, neon, and argon.
Summary
There are a total of 118 atoms in the periodic table. The first twenty elements of the periodic table is the first step towards mastering all of the elements. An element is a substance that can neither be oxidised nor reduced chemically. The elements’ atomic numbers follow the order in which they appear on the periodic table. We value the first 20 elements of the periodic table because they form the building blocks of all known life.
Without these in our systems, we just won’t make it. They are used as precursors in the synthesis of proteins, nucleic acid, and other biomolecules.
Frequently Asked Questions
1. What is the difference between a s-block element and a p-block element?
Ans: S-block elements are elements in the s-block of the periodic table, while p-block elements are elements in the p-block of the periodic table.
2. Are all the first elements of the periodic table non metals?
Ans: No, all the first elements of the periodic table are not non metals. Hydrogen and the p block elements are non metals while the s block elements are metals.
3. Are noble gases abundant in nature?
Ans: Every one of the noble gases exists in the atmosphere. Argon makes up 0.934% of the air we breathe, whereas the other 18 elements are present in extremely minute quantities. The radioactive decay of (\({potassium^ 40 }\)) is the primary source of atmospheric argon. In the atmosphere, neon makes up 0.0018 percent, helium 0.00052%, krypton 0.00011%, and xenon 0.000009%.
4. What is the periodic table, and why is it important?
Ans: The periodic table is a chart that organizes chemical elements by their atomic number, electron configurations, and recurring chemical properties. It is important because it allows scientists to predict the properties of elements and their compounds, making it an essential tool in the study of chemistry and the development of new materials.