Introduction
The electronic configuration describes the distribution of electrons within an atomic subshell. An electron configuration is a summary of the prediction of the position of the electrons surrounding a nucleus. In every neutral atom, the electron number is the same as the proton number. Now we’ll arrange those electrons so that they form a ring around the nucleus, displaying their energy and the orbital type in which they are located. Electrons occupy orbitals in a specific order based on their energy.
What do you understand by Electron Configuration?
- The electronic configuration describes the distribution of electrons within an atomic subshell.
- Atomic electronic configurations follow a standard format in which each atomic subshell containing an electron is listed in ascending order.
- For high atomic numbers, the standard representation of electronic configuration can be quite lengthy. In some cases, an abbreviated/condensed symbol may be used instead of the standard representation.
- The electron configuration of Na, for example, is \(1{s^2}2{s^2}2{p^6}3{s^1}\).
How Subshells are important for Electron Configuration?
- The azimuthal quantum no., represented by the letter “l,” determines the distribution of electrons into subshells.
- The magnitude of the principal quantum no., n, dictates the magnitude of this quantum number. As a result, when n equals 4, four distinct subshells can exist.
- For n = 4, the s, p, d, and f subshells correspond to l=0, 1, 2, 3 quantities.
- Equation 2(2l+1) gives the maximum number of electrons that a subshell can hold.
- The s, p, d, and f subshells can hold a maximum of 2, 6, 10, and 14 electrons, respectively.
Atomic Electronic Configuration Representation
This section provides examples of a few elements’ electronic configurations.
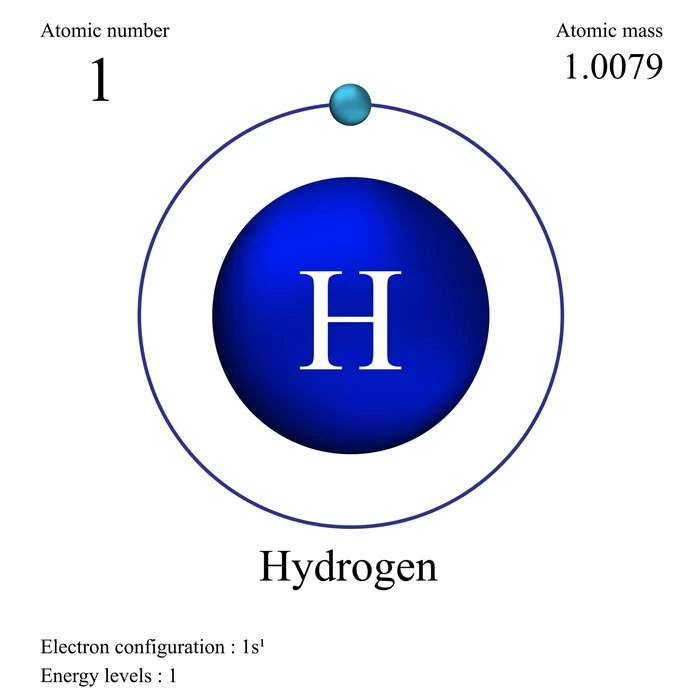
- The electron configuration of hydrogen has an atomic number of one. As a result, an H atom has one electron, which will be assigned to the subshell of the first shell/s orbit. \(1{s^1}\) is the electronic configuration of H.
- The electron configuration of chlorine
Cl has the atomic number 17. As a result, its 17 electrons are distributed as follows:
The K has two electrons.
The L has 8 electrons and the M has 7 electrons.
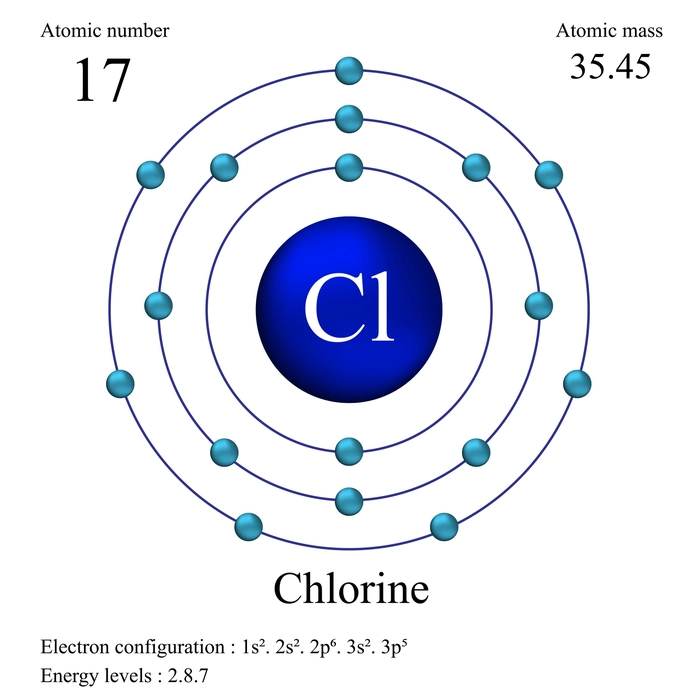
The electron configuration of Cl is depicted below. It is written as \(1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}\).
Filling Atomic Orbitals
The following concepts govern how electrons are occupied in atomic orbitals.
Aufbau Principle
“The energy of an atomic orbital is calculated by adding the principal and azimuthal quantum numbers, and according to the Aufbau principle, electrons begin in relatively low energy orbitals and progress to higher energy orbitals.”
Pauli Exclusion Principle
“Only electron pairs with opposite spins can be carried in an atomic orbital, and no two electrons in the same atom have the same values for all four quantum numbers. If two electrons have the same principle, azimuthal, and magnetic numbers, they should have opposing spins.”
Hund’s Law
“Before a second electron is placed in an orbital, each orbital in a specific subshell is said to be entirely filled by electrons.”
Summary
It can be concluded that Electron configuration is the depiction of electron distribution inside an element’s atomic shells. Because the electrons are mathematically positioned in these subshells, the configuration aids in determining their position. The periodic table categorises elements based on their electron configurations. These make up the s, p, d, and f-block elements. The maximum number of electrons that can fit in a shell is determined by the principal quantum number (n). The azimuthal quantum number, represented by the letter “l,” governs the distribution of electrons into subshells.
Frequently Asked Questions
1. Why are specific electron configurations required for elements?
Ans. Electron configurations can shed light on an atom’s chemical behaviour by identifying its valence electrons. It also aids in the organisation of elements into different blocks such as s, p, d, and f blocks.
2. Describe the significance of electron configuration.
Ans. The significance is as follows:
They aid in determining the reactivity state of an atom.
It aids in the identification of both chemical and physical properties.
It foretells an atom’s magnetic properties.
3. For n=3, which subshells are present?
Ans. Each orbital can hold a maximum of two electrons, and there are four subshells present- s, p, d, and f for n=3. The maximum number of orbitals corresponding to the s, p, d, and f subshells is 1,3,5, and 7.