Introduction
Acceleration represents the change in velocity of an object. It can either be positive or negative, depending on whether the final velocity is greater or less than the initial velocity. Thus, if the velocity of an object is decreasing with time, it is said to possess negative acceleration or retardation.
Acceleration is a vector quantity which has both magnitude as well as direction. During circular or rotational motion, the acceleration encountered is referred to as rotational acceleration. Note that it is possible for an object to have zero acceleration if it is moving with a constant velocity.
What is Acceleration?
In simplest of terms, acceleration is the rate of change of an object’s velocity with respect to time. Hence, its SI unit is given as \(m/{s^2}\).
General Formula of Acceleration
Generally, acceleration can be calculated via the following formula:
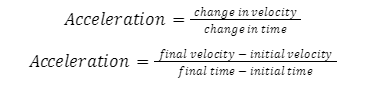
Acceleration dimensional Formula in Physics

Acceleration Unit

This is the SI unit of acceleration and other derived units can be found in different systems.
Acceleration Types
Most generally, acceleration can be classified into the following types:
- Uniform acceleration
- Non-uniform acceleration
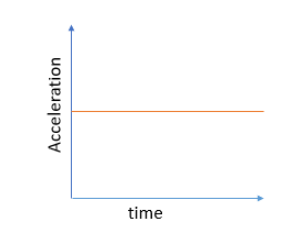
Uniform acceleration: An object whose velocity is changing at a constant rate is said to possess uniform acceleration.
Example: Suppose a car’s speed increases steadily by 30 m/s in 10s throughout a journey. That would mean that the car has a constant acceleration \(3m/{s^2}\).
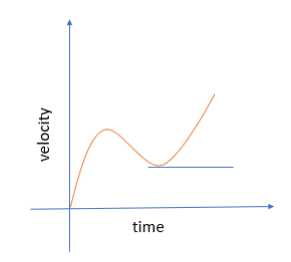
Non-uniform acceleration: Non-uniform acceleration is acceleration which itself does not remain steady in time.
Example: Suppose that during the first 2 hours of a journey, a car travels with a velocity of 15 km/hr. In the next 3 hours, the velocity changes to 45 km/hr. The change in velocity occurs over unequal intervals of time and thus, over the course of the journey, the car has non-uniform acceleration.
A few other types of acceleration may be stated as follows:
Acceleration due to gravity: This is simply the acceleration experienced by a body due to the gravitational force. Mathematically, it is equal to the gravitational force per unit mass experienced by the object.
Centripetal acceleration: A body in rotational motion experiences this type of acceleration and it is given as the centripetal force per unit mass experienced by a body undergoing circular motion.
Radial acceleration: Acceleration directed along the radius for a body in circular motion is called radial acceleration.
Angular acceleration: Angular acceleration is also experienced by a body in circular motion and its effect is to change the angular velocity of an object.
Coriolis acceleration: Coriolis force comes into the picture when the frame of reference we are considering is itself rotating with a given velocity. The coriolis acceleration is the acceleration encountered due to this coriolis force.
Average acceleration
For a non-uniform motion, we can find out the average acceleration of the object in question to get an overall sense of how it may have moved over the course of its journey. It is defined mathematically as follows.
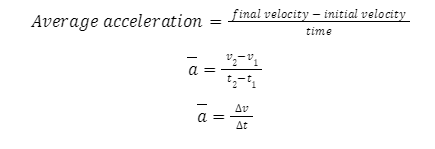
Instantaneous acceleration
The acceleration experienced by an object at a particular instant in time is referred to as instantaneous acceleration and it is given as the limiting value of the rate of change of velocity of an object when the time interval tends to zero.
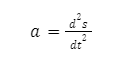
Where a= acceleration of the body, s=displacement of the body, and t= time. Thus, instantaneous acceleration is the second derivative of displacement.
Negative acceleration or retardation: Acceleration that slows down the motion of an object is referred to as retardation. For such a phenomenon to occur, the acceleration applied must be negative.
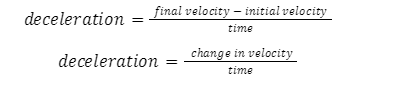
Velocity-Time Graph
Acceleration can also be derived from the velocity-time graph of an object. The slope of the graph gives us acceleration as a function of time and if we find the value of this slope at a particular point, i.e., the slope of tangent at a point, we arrive at instantaneous acceleration.
Graph for average acceleration
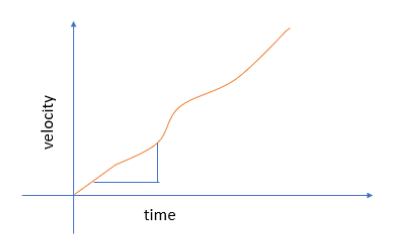
Graph for instantaneous acceleration
Graph for uniform acceleration
Graph for non-uniform acceleration
Examples of Acceleration
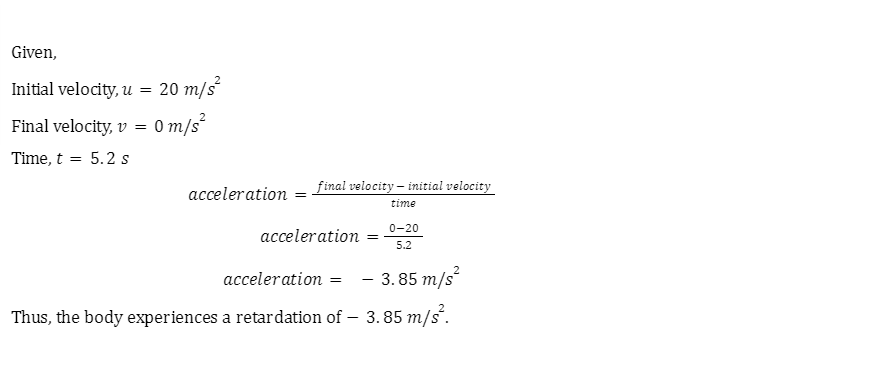
Example 1: A body moving with \(20m/{s^2}\) comes to a halt after 5.2 secs. Find the nature of acceleration and its value.
Solution:
Example 2: If a car is moving with a velocity of 45 m/s and after 10 s, its velocity becomes constant at 60 m/s, find acceleration.
Solution:

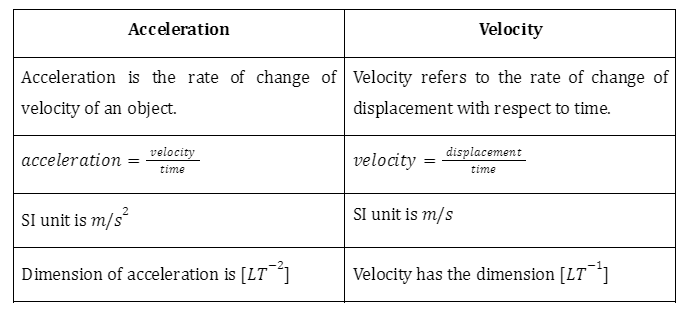
Difference between Acceleration and Velocity
Summary
Acceleration measures the change in velocity of an object. Its SI unit is \(m/{s^2}\) and it is a vector quantity with both magnitude and direction. It can be determined as the first-order derivative of velocity or the second-order derivative of position vector. An object moving with a constant velocity has zero acceleration since the derivative of a constant is equal to zero.
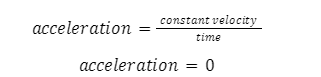
Frequently Asked Questions
1. Give two examples of retardation.
Two examples of retardation are as follows:
- A train that reaches a halt will slow down and thus, experience retardation.
- A ball thrown upwards experiences retardation due to gravity.
2. What is the SI and CGS unit of acceleration?
In SI units, acceleration is measured in \(m/{s^2}\) and in CGS units, in \(cm/{s^2}\).
3. What is gravitational acceleration?
Gravity is the force by which the Earth attracts a body towards its center. This force generates acceleration in a vertical motion, known as gravitational acceleration. The motion of an object falling solely under the effect of gravity is termed as free-fall.
4. What is the value of gravitational acceleration?
The approximate value of acceleration due to gravity is \(9.8m/{s^2}\).
5. What is angular acceleration?
Angular acceleration measures the time rate of change of angular velocity of an object and thus, is measured in \(rad/{s^2}\).
6. What is a Coriolis force?
Coriolis force is experienced by objects which are moving in a frame of reference that is itself rotating with a given angular velocity. It is responsible for wind in certain regions of the Earth.