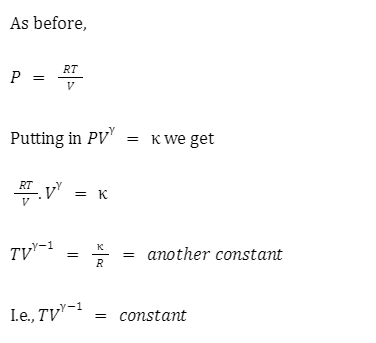Introduction
At present, bioremediation has been recognized as one of the most effective methods for cleaning up contaminated soil and water. As a natural and sustainable technology, bioremediation can save costs, reduce environmental impact, and promote healthy living conditions. In this article, we will explore the benefits and challenges of bioremediation and provide you with insights into how bioremediation can be used to address the growing concerns about soil and water pollution.
What is Bioremediation?
Bioremediation is a process that uses microorganisms to break down, neutralize, or remove contaminants from polluted soil and water. The microorganisms used in bioremediation can be naturally occurring or genetically modified, and they can degrade organic or inorganic contaminants, such as petroleum, pesticides, herbicides, and heavy metals. Bioremediation can be carried out in situ, which means that the contaminated soil or water is treated on-site, or ex-situ, which means that the contaminated soil or water is removed from the site and treated in a controlled environment.
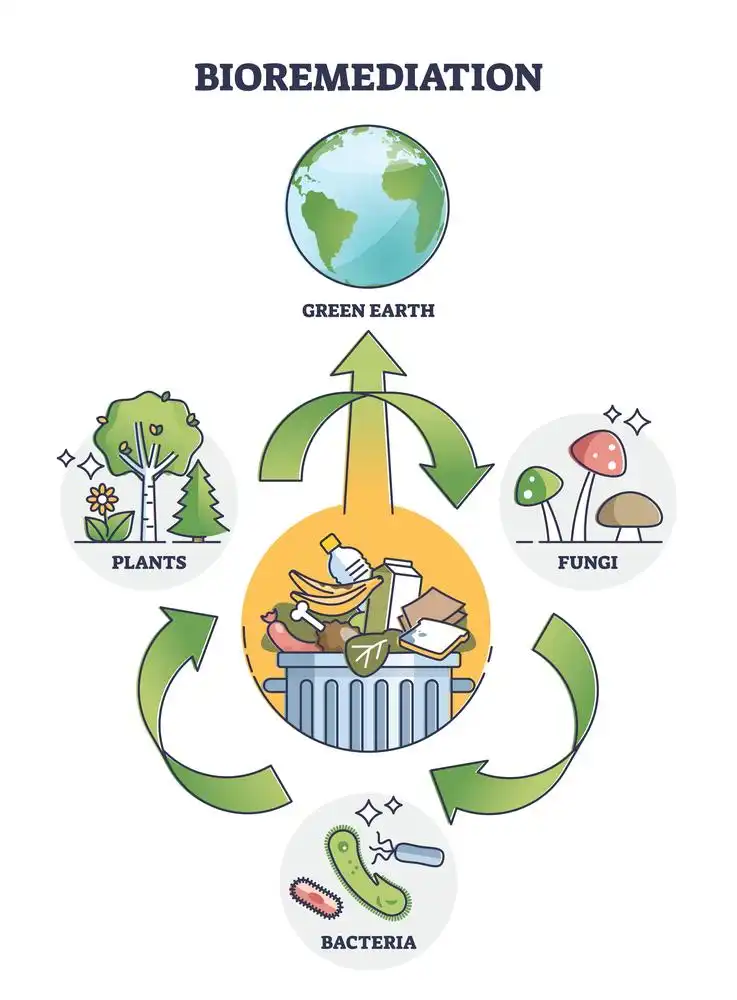
Bioremediation
Types of Bioremediation
- Phytoremediation: This type of bioremediation uses plants to remove contaminants from the environment. Plants absorb pollutants through their roots, which are then broken down by bacteria in the soil. Phytoremediation is often used to treat contaminants such as heavy metals, organic compounds, and petroleum.
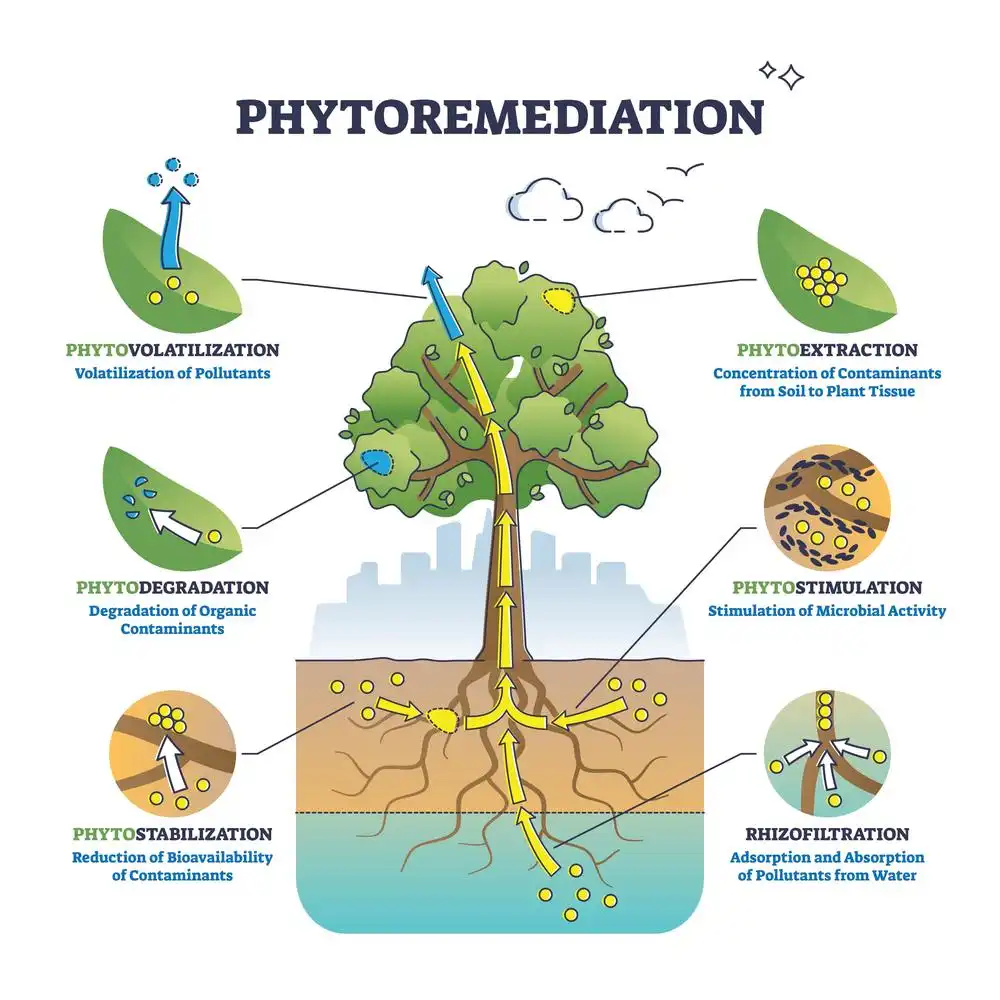
Phytoremediation
- Bioaugmentation: Bioaugmentation involves the addition of microbes to contaminated sites to enhance their ability to break down pollutants. Microbes such as bacteria, fungi, and algae are added to the contaminated area, where they consume the pollutants and convert them into less harmful substances.
- Biostimulation: Biostimulation involves adding nutrients, oxygen, or other substances to the contaminated site to promote the growth of naturally occurring microbes. This process enhances the ability of the existing microbial community to break down pollutants.
- Bioventing: Bioventing is a process that involves the injection of air or oxygen into contaminated soil to promote the growth of aerobic microbes. These microbes break down pollutants into less harmful substances such as carbon dioxide and water.
- Composting: Composting is a type of bioremediation that involves the breakdown of organic pollutants by bacteria and other microorganisms. Organic waste, such as food waste or agricultural waste, is mixed with other materials such as wood chips, leaves, and grass clippings to create a compost pile. The microbes in the compost pile break down the organic waste and convert it into nutrient-rich soil.
Bioremediation Process
The bioremediation process involves several steps, including:
- Site Assessment: The first step in the bioremediation process is to assess the contaminated site. This involves collecting soil and water samples to determine the type and extent of the contamination.
- Selection of Bioremediation Technique: Once the site has been assessed, the most appropriate bioremediation technique is selected based on the type and extent of contamination, as well as other factors such as cost and time constraints.
- Preparation of Site: The contaminated site is prepared for bioremediation, which may involve the removal of debris or other obstacles that may hinder the process.
- Application of Bioremediation Technique: The selected bioremediation technique is applied to the contaminated site, which may involve the addition of microbes, nutrients, or other substances to the soil or water.
- Monitoring: The bioremediation process is monitored to ensure that the pollutants are being broken down and that the environmental conditions are suitable for the growth of the microbes.
- Completion: Once the pollutants have been sufficiently broken down, the bioremediation process is considered complete.
The Benefits of Bioremediation
Bioremediation offers several benefits over traditional methods of pollution control. Some of these benefits include:
- Natural and Sustainable: Bioremediation is a natural process that uses living organisms to break down and degrade pollutants. It is a sustainable approach to pollution control and does not rely on the use of chemicals or heavy machinery.
- Cost-Effective: Bioremediation is a cost-effective method of pollution control. It requires fewer resources and is less expensive than traditional methods such as excavation and incineration.
- Versatile: Bioremediation can be used to clean up a wide range of pollutants, including oil spills, heavy metals, and organic chemicals.
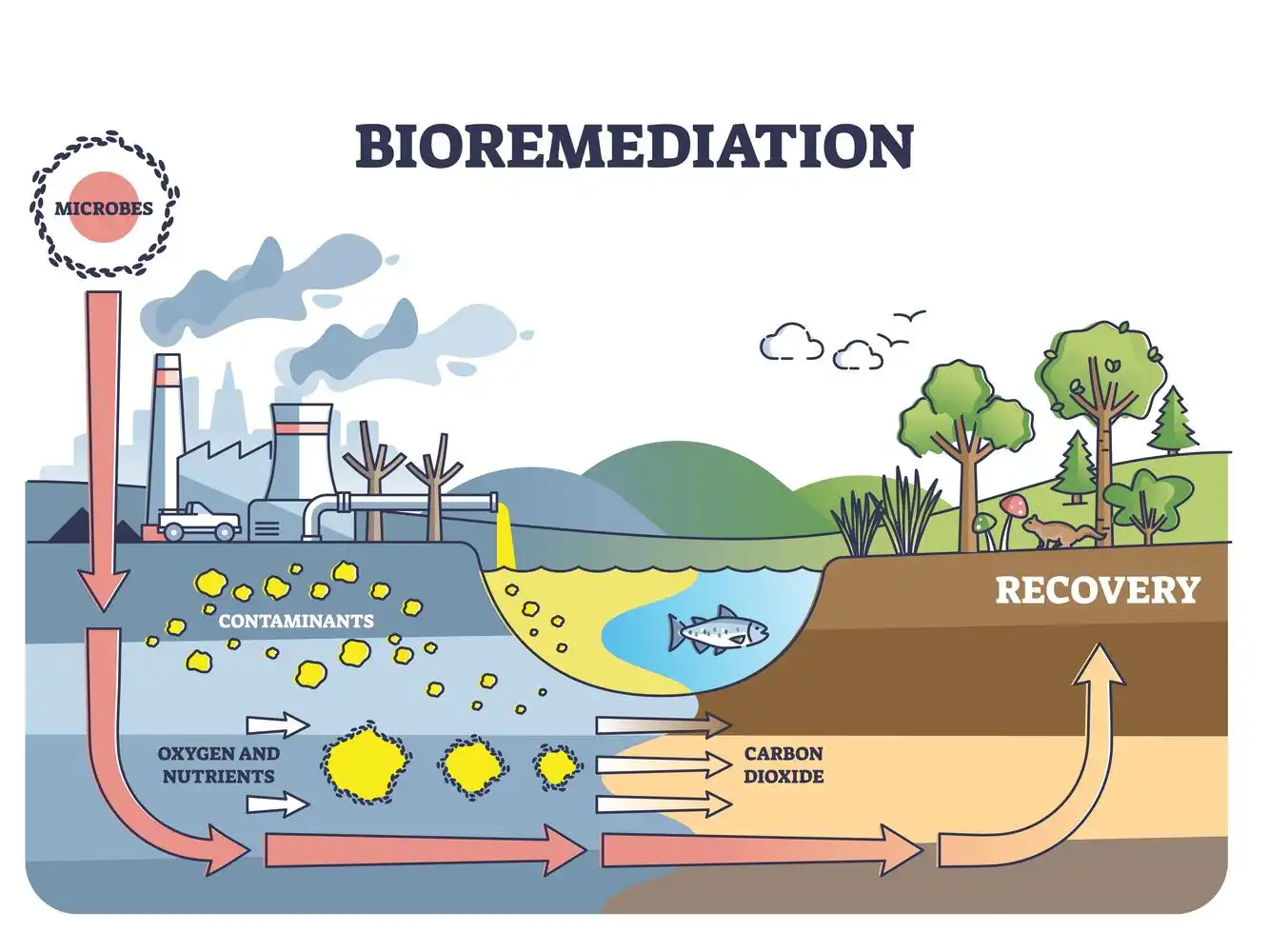
Recovery of the ecosystem by bioremediation
The Challenges of Bioremediation
Although bioremediation has many benefits, there are also some challenges associated with this technology. The success of bioremediation depends on several factors, including the type and concentration of contaminants, the characteristics of the soil or water, the availability of nutrients and oxygen, and the temperature and pH of the environment. In some cases, bioremediation may not be effective due to the lack of suitable microorganisms, or the presence of inhibitors that prevent the microorganisms from degrading the contaminants. Moreover, bioremediation can take a long time to complete, and it may require multiple treatments over a period of several months or years.
The Applications of Bioremediation
Bioremediation has been used to address a wide range of contaminated sites, including industrial, agricultural, and residential areas. Some examples of bioremediation applications are:
- Petroleum spills: Bioremediation has been used to clean up oil spills in marine and terrestrial environments. The microorganisms used in bioremediation can break down the hydrocarbons in the oil and convert them into harmless byproducts, such as water and carbon dioxide.
- Agricultural runoff: Bioremediation has been used to reduce the levels of pesticides and herbicides in agricultural runoff. The microorganisms used in bioremediation can degrade the chemicals and prevent them from reaching the groundwater or surface water.
- Landfills: Bioremediation has been used to reduce the levels of organic and inorganic contaminants in landfills. The microorganisms used in bioremediation can break down the contaminants and reduce the volume of waste.
Conclusion
In conclusion, bioremediation is a valuable tool in the cleanup of contaminated sites, and several types of bioremediation techniques can be used. The bioremediation process involves several steps, and each step is crucial to the success of the process. With careful planning and execution, bioremediation can help to restore contaminated sites to a healthier state.
Frequently Asked Questions
1. What are the disadvantages of bioremediation?
Bioremediation may not be effective for all types of pollutants, and the process can be slow.
2. Is bioremediation safe?
Bioremediation is a safe and environmentally friendly approach to pollution control. However, it is essential to ensure that the process is properly managed to prevent any unintended consequences.
3. How much time may bioremediation require?
The average length of the bioremediation process can range from several months to several years, depending on factors such as the size of the polluted region, the number of toxins present, the temperature, the density of the soil, and whether ex-situ or in situ bioremediation is used.




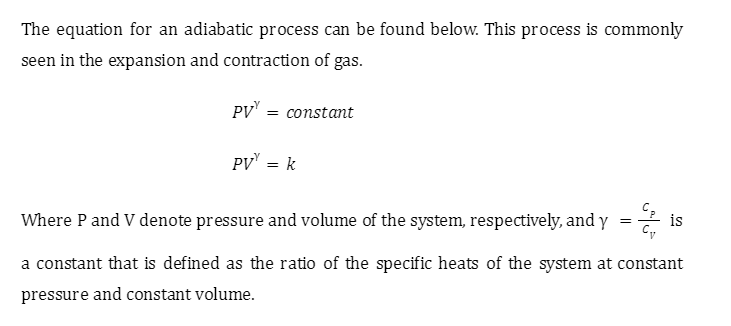
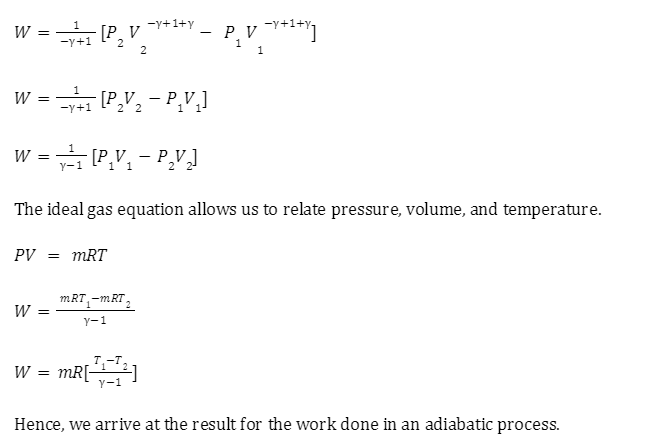
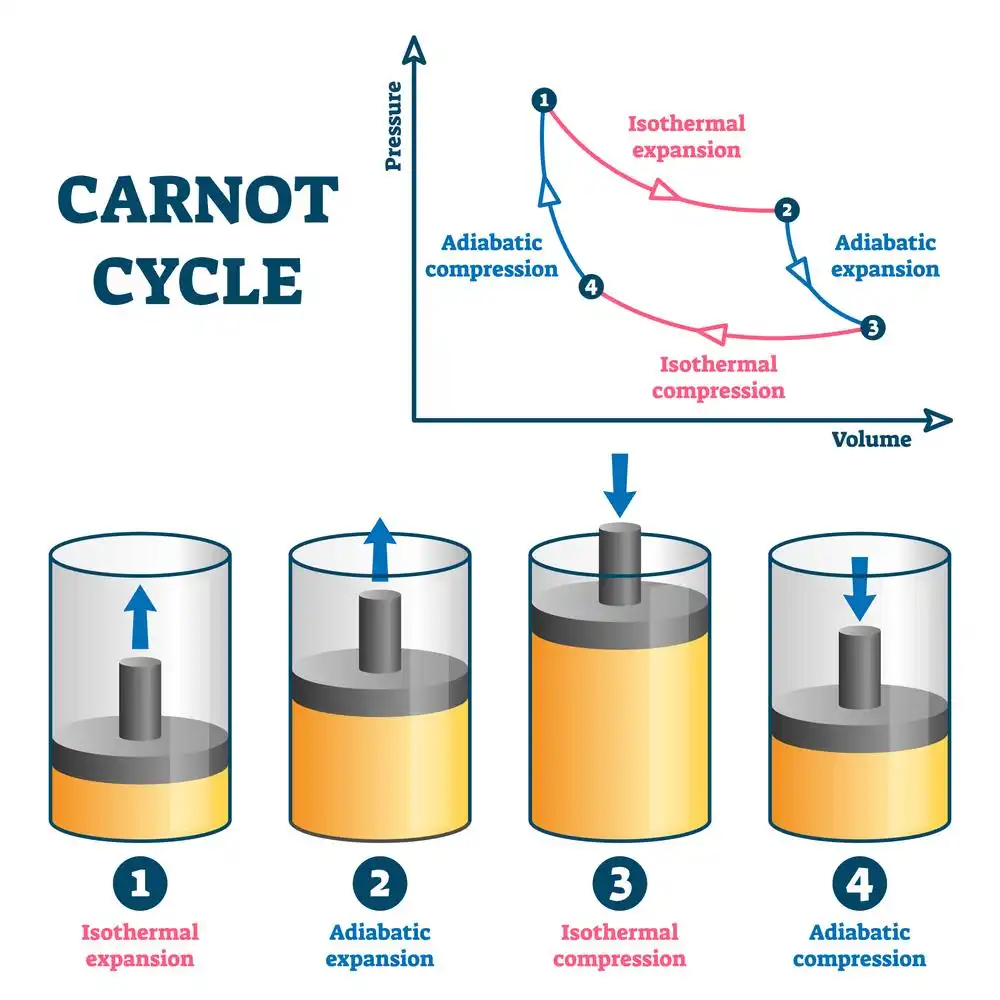
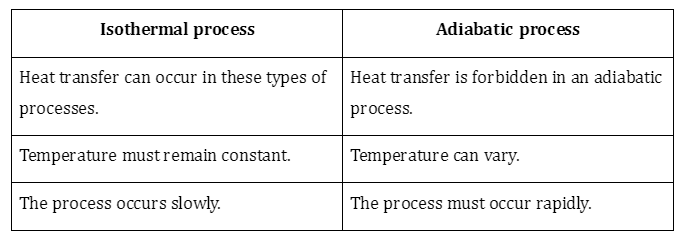
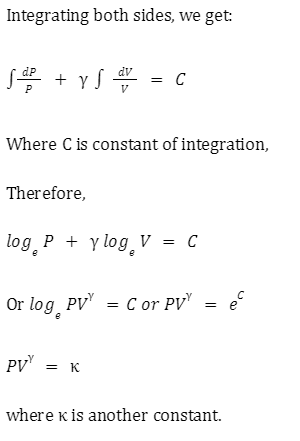
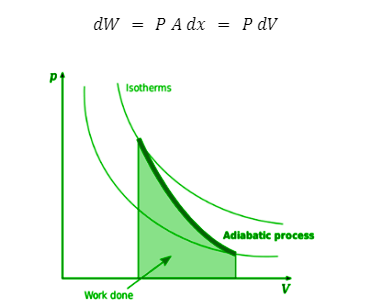
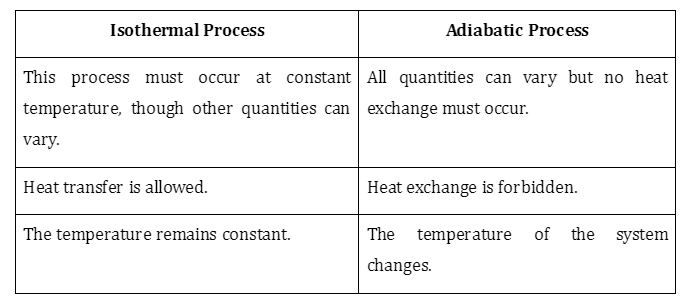
 Isotherms and adiabats
Isotherms and adiabats