Introduction
Under the right circumstances, fire can be a very helpful chemical reaction. It’s useful in a lot of situations, but it can be dangerous if it’s burned improperly. The portable gadget known as a fire extinguisher is used to put out fires of any size. People depend on fire for a wide variety of tasks. Fire is essential for many industrial processes; without it, it would be impossible to imagine things like cooking and lighting. Dry vegetation in woods has caught fire before, posing a threat to wildlife for miles around. Multiple types of fire extinguishers are used to put out the various blazes. Many various kinds of fire extinguishers are available, including those that use water and foam, carbon dioxide, dry chemicals, wet chemicals, water mist, and so on.
What is a Fire Extinguisher?
To put out a fire, you need an extinguisher, which can contain dry carbon, water, or a chemical. It’s put to use dousing flames caused by things like cooking oil, flammable gases, petroleum, wood, clothing, paint, and so on. These are stashed in convenient, easy-to-reach locations. Classifications of fire depend on the nature of the combustible substance.

Fire Extinguisher
Explain the Principle on which a Fire Extinguisher works
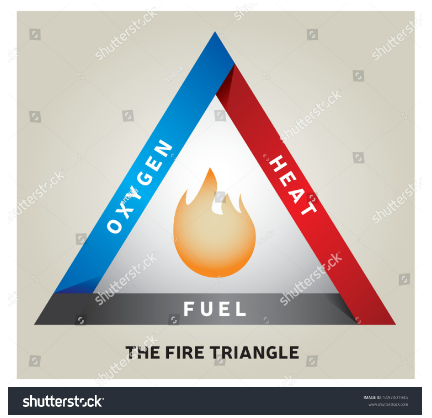
A fire extinguisher relies on the “fire triangle,” a set of interrelated concepts for its operation.
- When fighting a fire, the primary rule is to extinguish it at its origin.
- The availability of oxygen is the second essential item.
- The third component is the fuel being used in the fire.
Fire Triangle
Types of fire extinguisher
The fire extinguisher is of numerous varieties, since the fire extinguisher acts according to the source of the fire. There are seven distinct varieties of fire extinguisher, each distinguished by the chemical it contains.
- Water and foam Based: Electric appliance, coal, paper, textile, wood, etc. fires can all be put out with a foam base fire extinguisher. Use a water-based extinguisher for flames involving metal, wood, cooking grease, and similar materials.
- Carbon Dioxide Based: In this type of extinguisher contains carbon dioxide, which hinders the supply of oxygen and helps cool down fire. This is used for fires caused by electricity.
- Wet Chemical-based Extinguisher: This type of extinguisher is used for the fire caused by oils, fats, and in commercial kitchens. It removes the heat based on the fire triangle principle.
- Dry Powder Fire Extinguisher: A dry powder is filled in an extinguisher; it hinders the supply of oxygen to cool down the heat generated. It is used in fire caused by metals, like sodium, zirconium, etc.
- Clean Agent Fire Extinguisher: It contains a halogenated clean agent i.e., halogen with ozone-depleting hydrocarbons.
- Water Mist Extinguisher: They are used to quench fires caused by wood, paper, as well as electric appliances.
- Dry Chemical Fire Extinguisher: It is filled with a dry chemical that interrupts the chemical reaction that is the cause of the fires
Working of Water Fire Extinguisher
- Water extinguishers are filled with water and designed in such a way that when the seal is broken it expels the water in force to quench a fire.
- First, the seal is broken, and the safety pin is pulled out.
- Then, the lever of the extinguisher is squeezed.
- By squeezing the lever, it forces a pointed rod within the valve, that punctures the cylinder containing high-pressure gas.
- The Gas-filled in the cylinder is released into the cylinder filled with water and, forces the water downward.
- Pressured water then came out of the pipe, this pressure triggered water to cool down the fire from 4 to 6 feet away.
Preparation of Soda Acid Fire Extinguisher with diagram and explain How it works?
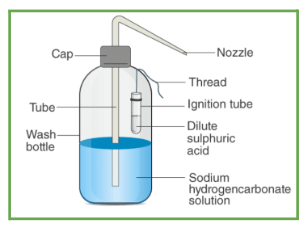
The Soda acid fire extinguisher is prepared with sodium bicarbonate and diluted sulphuric acid. Let’s check the process of preparation of a Soda acid fire extinguisher.
- First, we need a wash bottle with a nozzle, we fill that bottle with 20 ml of sodium bicarbonate \(\left( {NaHC{O_3}} \right)\) solution,
- Then, we suspend an ignition tube by a thread that contains a dilute solution of sulphuric acid \(\left( {{H_2}S{O_4}} \right)\) in the wash bottle,
- The next step is to close the mouth of the bottle,
- After closing the bottle, we tilt the bottle in such a way that the acid-filled ignition tube reacts with the sodium bicarbonate solution,
- After some time, we notice that there is discharge coming out of the nozzle of the bottle.
- That discharge is the of carbon dioxide \(C{O_2}\) , and other products are sodium sulphates and water.
- When we take the discharge near the fire it quenches the supply of oxygen within the fire and the fire cools down.
- The Carbon dioxide \(C{O_2}\) released during the reaction work as an extinguisher that hinders the supply of oxygen in fire and hence fire cools down.
\({\bf{2NaHC}}{{\bf{O}}_3} + {{\bf{H}}_2}{\bf{S}}{{\bf{O}}_4} \to {\rm{ }}{\bf{N}}{{\bf{a}}_2}{\bf{S}}{{\bf{O}}_4} + {\bf{2}}{{\bf{H}}_2}{\bf{O}} + {\bf{2C}}{{\bf{O}}_2}\)
Preparation of Soda Fire Extinguisher
Summary
In the above tutorial, we have studied the fire extinguisher. A fire extinguisher is a container that contains different types of elements like foam, wet chemical, dry chemical, carbon dioxide, water, water mist, etc. we must choose a correct fire extinguisher when a fire breaks out because a wrong type of extinguisher can ignite the fire more despite cooling it down. The fire is divided into five classes class A, B, C, D, and K. These are divided according to the type of material causing the fire i.e., wood, paper, electric appliance, oil, fat, metals, etc. These types of fires are quenched by different types of fire extinguishers known as Foam, water, chemical, and carbon dioxide-based extinguishers.
Frequently Asked Questions:
1. What is the difference between a rechargeable and a non-rechargeable fire extinguisher?
Ans. Rechargeable fire extinguishers can be refilled and reused, while non-rechargeable fire extinguishers must be replaced after use.
2. How long does a fire extinguisher last?
Ans. The lifespan of a fire extinguisher depends on the type of extinguisher and the environment in which it is stored. Generally, fire extinguishers should be replaced every 5-10 years.
3. What is the best way to store a fire extinguisher?
Ans. Fire extinguishers should be stored in a cool, dry place away from direct sunlight and away from any heat sources.