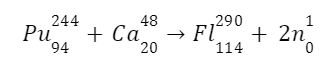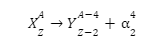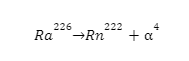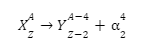Introduction
The chemical symbol for flerovium is Fl, and the atomic number of flerovium is 114. Super heavy describes this element’s status as one of the heaviest in the periodic table.
It’s also a highly radioactive substance or component. It wasn’t until 1998, in a Russian research facility, that this element was found.
The p-block is represented by elements in the periodic table by those in group 14, period 7. In its electronic form, flerovium has the formula\(\left[ {{\bf{Rn}}} \right]{\rm{ }}{\bf{5}}{{\bf{f}}^{14}}{\bf{6}}{{\bf{d}}^{10}}{\bf{7}}{{\bf{s}}^2}{\bf{7}}{{\bf{p}}^2}\). Since it is the most massive element in the carbon (C) family, it is a member of that family. Its radioactivity means that only a little amount of this element may ever be produced.

What is Flerovium?
With an atomic number of 114 and the symbol, flerovium is one of the heaviest known elements (Fl). To be specific, this element belongs to the p-block. It is part of period 7 and the 14th group. Then it must be one of the elements of the carbon group. It’s also the heaviest member of the carbon family. As a man-made substance, it is also highly (very) radioactive (does not occur naturally on the surface of the earth).
At the time of its discovery in 1998, the element was given the name flerovium in honour of the flerov laboratory of nuclear reactions in Dubna, Russia (Asia). However, over time, the name was changed to the flerov to honour the Russian physicist Georgy Flyorov. According to estimates, its mass will be 289.
How Scientists Discovered Flerovium Elements?
In 1998, researchers at Russia’s Joint Institute for Nuclear Research in Dubna successfully synthesised flerovium. They pounded plutonium atoms with calcium ions.
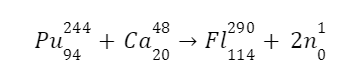
One atom of flerovium-289, an isotope with a half-life of around 21 seconds, was created in this process.
What are the Uses of Flerovium?
Due to it being the heaviest element and the radioactive, Flerovium has very few applications. This substance has zero practical applications outside of academic institutions. No biological function is served by it Its formation in nuclear reactors is possible. Very few flerovium atoms have been created or identified so far. Because of its hazardous properties, it is also not used in commercial applications (as it is a radioactive element).
Flerovium Chemical Properties
- Flerovium is the bulky member present in group 14 elements found below lead.
- The chemical behaviour of the element is expected to be very distinctive.
- The 7s orbitals (s, p, d, f…) are very (largely) highly (more) stabilized (stable) in flerovium, therefore a very large \(s{p^3}\) (type) orbital (orbits) hybridization is needed to achieve a +4-oxidation state (O.S.).
- Flerovium is predicted to be more stable (most) than lead (Pb) in its +2-oxidation state while at a +4 (O.S.)-oxidation state it should be highly (very much) unstable.
Physical Properties of Flerovium
Some of the physical properties of Flerovium element are as follows-
- Appearance- Liquid phase at STP (predicted)
- Melting Point (M.P.)- It has a melting point of around 200K or -73 °C or -100 °F.
- Boiling Point (B.P.)- It has a boiling point of around 380K or 107 °C or 224°F.
- Density- It has a density of approximately 9.928 \(g/c{m^3}\).
- Crystal Structure- Flerovium has a face-centred cubic lattice type.
General Properties of The Fl Element
Flerovium is a rarely used heavy material. Its atomic number is 114 and the molar mass is predicted to be 289. It is denoted or represented by the symbol Fl. It is a carbon group element that lies in the p-block of the periodic table. Its colour is predicted (assumed) to be silvery-white, grey, or metallic. Sometimes it is also known as Ununquadio. Flerovium is a radioactive synthetic element with five known isotopes so far.
Fun Facts About the Fl Element
- Flerovium is considered a metal due to its high density and it is an extremely radioactive metal.
- This metal or element can be rarely produced in nuclear reactors.
- Its production is very expensive.
- It is obtained by bombarding plutonium ions and calcium.
- It is a p-block element present in the carbon group.
Summary
Flerovium represented or denoted by the symbol (Fl) is one of the heaviest known elements with the atomic number (Z) 114. It is a p-block element. It lies in group 14 and period 7. That means it is a carbon group element. And it is the heavier element in the carbon group and the p-block. It is also an extremely (very much) radioactive man-made element (does not occur naturally on the surface of the earth). At the time of discovery, it was named after (the place where it was produced) flerov laboratory (lab) of nuclear reactions located in Dubna, a place in Russia, where this element (metal) was produced (discovered) in the year 1998. Element is produced by bombarding a target (aim) of plutonium-244 (\(Pu_{94}^{244}\)) with the nuclei (accelerated) of calcium (\(Ca_{20}^{48}\)):
Frequently Asked Questions
1. How many isotopes of Flerovium are known?
Ans: Flerovium has seven known isotopes, and possibly 2 nuclear isomers. The longest-lived isotope is \({}^{289}Fl\) with a half-life of 1.9 seconds, but the unconfirmed \({}^{290}Fl\) may have a longer half-life of 19 seconds.
2. Who discovered flerovium and when?
Ans: A team of scientists from JINR (Joint Institute for Nuclear Research), located in Dubna, a place in Russia discovered the flerovium (element) in the year 1998.
3. Write the reaction involved in the formation of flerovium.
Ans: The reaction involved in the formation of flerovium is –