Introduction
Alcohols and phenols are formed by replacing one old hydrogen atom in the hydrocarbon. In the case of alcohols, the -OH is known as the hydroxyl group, while it is called the phenolic group. It is attached to a benzene ring. Since they have a common functional group, most of the characteristics of alcohols and phenols are expected to be the same. However, they do differ in many properties. It is primarily due to the reason that the phenolic group is involved in residence or conjugation with the benzene ring. While no search resonance is possible in alcohols. This makes phenols considerably acidic while alcohols hardly exhibit any acidic in nature. Members of both these families have analytical and industrial importance.
Structure of Alcohol
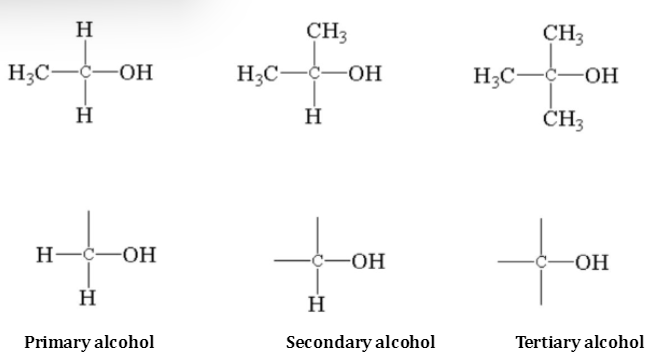
Alcohols which contain one -OH group that is monohydric compounds are also known as alcohols. These are represented by the formula R-OH These are additionally categorised as primary (1°), secondary (2°) and tertiary (3°) varying on the behaviour of the carbon atoms to which the -OH group is bonded. For example,
Structure of Phenol
Phenols are aromatic compounds that contain one or more, -OH groups called phenolic groups that are attached directly to the ring. Phenols may be further classified as monohydric, dihydric, trihydric, etc, depending upon the number of -OH, groups bonded to the ring.
Monohydric phenols known as the parent or simplest member of the family with one -OH group attached to the ring are called phenol.

In dihydric phenolic compounds 2 -OH, groups are attached directly to the ring.

Trihydric phenols compounds are known in which 3 -OH groups are directly attached to the ring

Difference Between Hydroxyl and Alcohol

What is Hydroxyl?
The term hydroxyl is used to refer to the -OH radical. A functional group found in both organic and inorganic compounds is the hydroxyl group. The chemical formula for this compound is -OH. As a result, the hydroxyl group is made up of one hydrogen and one oxygen atom. The hydroxyl radical is extremely reactive and can initiate chemical reactions almost instantly. The Hydroxyl radical is the Hydroxide ion’s neutral form OH–. This hydroxyl radical has an unpaired electron, which results in the radical’s high reactivity.
What is the Relationship Between Hydroxyl and Alcohol?
Alcohols are composed of the -OH groups. These -OH groups behave as the functional group of alcohols. Consequently, the -OH group affects the physical and chemical properties of alcohols.
Because of -OH group presence alcohols tend to show some properties that are only because of the -OH group.
- They are polar compounds directly because of the presence of -OH groups.
- Alcohols are soluble in polar solvents.
- Alcohols have the ability to form Hydrogen bonds.
- Alcohols are soluble in water.
- Alcohols tend to have higher boiling points than the equivalent alkanes because of the existence of these hydrogen bonds.
Summary
Alcohols and phenols are formed by replacing one or more hydrogen atoms in hydrocarbons by -OH groups. In the case of alcohols, the -OH group is known as the hydroxyl group, while it is called the Phenolic group when connected to a benzene ring. Given that they have the same functional group, most of the properties of alcohols and phenols are believed to be similar. Nevertheless, they do not differ in many properties, it is mainly because of the explanation that the phenolic group is engaged in resonance or conjugation with the benzene ring. However, no such resonance is feasible in alcohols. This makes phenols significantly acidic, while alcohols barely demonstrate any acidic character. Members of both these families have analytical and industrial significance. Ethyl alcohol, commonly called alcohol, is a starting material for the manufacture of ether, chloroform, acetic acid, etc. It can also be used as a fuel for spirit lamps and stoves due to its highly combustible nature. But it’s important, is in its ability to act as a beverage in the form of beer, wine, whisky, Brandy, etc. phenol finds application in the synthesis of Bakelite, plastics, drugs, etc.
Frequently Asked Questions
1. Out of Phenols and Alcohols, which one is more acidic in nature and why?
Phenols are more acidic in nature because of the explanation that the phenolic group is engaged in resonance or conjugation with the benzene ring. However, no such resonance is feasible in alcohols. This makes phenols significantly acidic, while alcohols barely demonstrate any acidic character.
2. Name the tests to distinguish between primary, secondary and tertiary
Alcohol?
In the chemical properties of alcohols, we have seen that the three types of alcohols differ in the nature of their products. However, they cannot be distinguished practically based on these characteristics. Special tests are employed for this purpose. These are described as follows.
Victor Meyer’s Test (Red-blue Colourless Test)
- In this, a blood-red colouration indicates primary alcohol.
- A blue colouration indicates secondary alcohol
- A colourless solution represents tertiary alcohol
Lucas Reagent Test
- If turbidity appears immediately, alcohol is tertiary
- If turbidity appears after some time, alcohol is secondary
- In case turbidity appears on heating, alcohol is primary
3. Describe Lucas Reagent test? Why is it important? What are its limitations?
This test is based on the reactivities of primary secondary and tertiary alcohols with Hydrochloric acid. The given alcohols are treated with Lucas reagent, which is an equal mixture of concentrated HCl and anhydrous \(ZnC{l_2}\), which is a dehydrating agent. The product is alkyl chloride or chloroalkane accompanied by white, turbidity or cloudiness.

The time taken for the appearance of turbidity is different in the 3 types of alcohols and affords a method for their distinction.
- If the turbidity appears immediately, alcohol is tertiary
- If the turbidity appears after sometime, alcohol is secondary
- In case the turbidity appears on heating, alcohol is primary
Limitations of Lucas reagent.
Lucas reagent test is not applicable to the alcohols with 6 or more carbon atoms. As they are not water soluble, no reaction with Lucas reagent is possible.
- Describe the physical properties of Phenols?
- State and smell. Phenols are either colourless crystalline solids or liquids. However, when exposed to the atmosphere, they become reddish or pinkish due to the formation of oxidation products. Phenols have a characteristic smell, known as phenolic smell.
- Solubility. I like alcohol. Phenols are only sparingly soluble in water. They are also expected to form hydrogen bonding with water molecules due to the polar nature of the -OH group present.
- Boiling points. Phenols are expected to have higher boiling points than expected from their molecule formula, mainly because of the polar nature of the -OH group. Thus, these are having higher boiling points than the aromatic hydrocarbons of comparable molecular masses.
5. What will happen when alcohol is oxidised?
Oxidation of primary alcohols to aldehyde
Aldehydes are the oxidation products of primary alcohols.

Oxidation of secondary alcohols to Ketones
Ketones are products of oxidation of secondary alcohols.